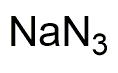Sodium azide 0.1 M solution is widely utilized in research focused on:
- Laboratory Reagent: Commonly used as a preservative in biological and chemical laboratories, it helps maintain the stability of samples by preventing microbial growth.
- Explosive Material: In controlled environments, it serves as a precursor in the synthesis of azide-based explosives, valued for their high energy output and rapid decomposition.
- Biochemical Research: Employed in various biochemical assays, it acts as a potent inhibitor of cytochrome c oxidase, making it useful for studying mitochondrial functions and cellular respiration.
- Pharmaceutical Development: Utilized in the synthesis of pharmaceuticals, particularly in the development of drugs targeting cardiovascular diseases due to its ability to release nitrogen gas under certain conditions.
- Safety Applications: Used in the production of safety devices, such as airbags in vehicles, where its rapid decomposition generates gas to inflate the airbag quickly upon impact.
General Information
Properties
Safety and Regulations
Applications
Sodium azide 0.1 M solution is widely utilized in research focused on:
- Laboratory Reagent: Commonly used as a preservative in biological and chemical laboratories, it helps maintain the stability of samples by preventing microbial growth.
- Explosive Material: In controlled environments, it serves as a precursor in the synthesis of azide-based explosives, valued for their high energy output and rapid decomposition.
- Biochemical Research: Employed in various biochemical assays, it acts as a potent inhibitor of cytochrome c oxidase, making it useful for studying mitochondrial functions and cellular respiration.
- Pharmaceutical Development: Utilized in the synthesis of pharmaceuticals, particularly in the development of drugs targeting cardiovascular diseases due to its ability to release nitrogen gas under certain conditions.
- Safety Applications: Used in the production of safety devices, such as airbags in vehicles, where its rapid decomposition generates gas to inflate the airbag quickly upon impact.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.