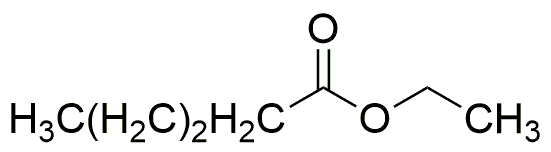
Ethyl valerate is widely utilized in research focused on:
- Flavoring and Fragrance Industry: This compound is commonly used as a flavoring agent in food products and beverages, providing a fruity, pleasant aroma. Its natural appeal makes it a preferred choice over synthetic alternatives.
- Pharmaceutical Applications: Ethyl valerate serves as an intermediate in the synthesis of various pharmaceuticals, enhancing the efficacy of active ingredients and improving drug formulation stability.
- Perfume Manufacturing: In the cosmetic industry, it is used in the formulation of perfumes and personal care products, contributing to the overall scent profile and enhancing the product's marketability.
- Solvent in Chemical Reactions: It acts as a solvent in organic synthesis, facilitating reactions due to its favorable properties, which can lead to increased yields and reduced reaction times.
- Research and Development: Ethyl valerate is utilized in academic and industrial research for studying esterification reactions and evaluating the properties of esters, providing insights into chemical behavior and potential applications.
General Information
Properties
Safety and Regulations
Applications
Ethyl valerate is widely utilized in research focused on:
- Flavoring and Fragrance Industry: This compound is commonly used as a flavoring agent in food products and beverages, providing a fruity, pleasant aroma. Its natural appeal makes it a preferred choice over synthetic alternatives.
- Pharmaceutical Applications: Ethyl valerate serves as an intermediate in the synthesis of various pharmaceuticals, enhancing the efficacy of active ingredients and improving drug formulation stability.
- Perfume Manufacturing: In the cosmetic industry, it is used in the formulation of perfumes and personal care products, contributing to the overall scent profile and enhancing the product's marketability.
- Solvent in Chemical Reactions: It acts as a solvent in organic synthesis, facilitating reactions due to its favorable properties, which can lead to increased yields and reduced reaction times.
- Research and Development: Ethyl valerate is utilized in academic and industrial research for studying esterification reactions and evaluating the properties of esters, providing insights into chemical behavior and potential applications.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.