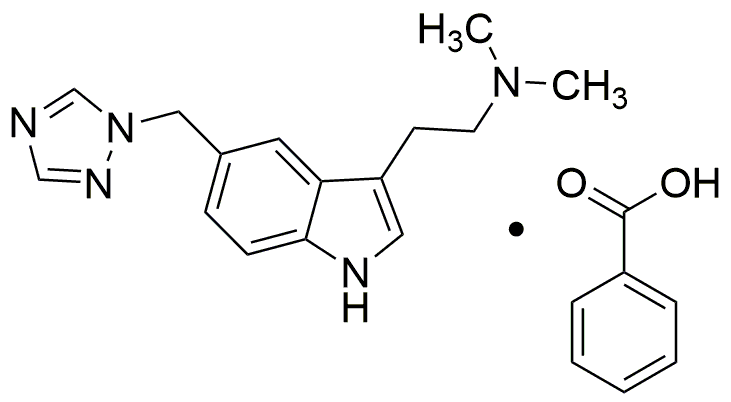
Rizatriptan benzoate is widely utilized in research focused on
- Migraine Treatment: This compound is primarily used in the pharmaceutical industry for the acute treatment of migraines, providing rapid relief to patients by targeting serotonin receptors in the brain.
- Clinical Trials: It plays a significant role in clinical research, helping to evaluate the efficacy and safety of new formulations or combinations with other medications aimed at headache disorders.
- Pharmacokinetic Studies: Researchers utilize it to study the absorption, distribution, metabolism, and excretion (ADME) properties, which are crucial for developing effective dosing regimens.
- Formulation Development: The compound is involved in the development of various dosage forms, including tablets and orally disintegrating formulations, enhancing patient compliance and convenience.
- Comparative Effectiveness Research: It is used in studies comparing the effectiveness of different migraine treatments, helping healthcare providers make informed decisions about patient care.
General Information
Properties
Safety and Regulations
Applications
Rizatriptan benzoate is widely utilized in research focused on
- Migraine Treatment: This compound is primarily used in the pharmaceutical industry for the acute treatment of migraines, providing rapid relief to patients by targeting serotonin receptors in the brain.
- Clinical Trials: It plays a significant role in clinical research, helping to evaluate the efficacy and safety of new formulations or combinations with other medications aimed at headache disorders.
- Pharmacokinetic Studies: Researchers utilize it to study the absorption, distribution, metabolism, and excretion (ADME) properties, which are crucial for developing effective dosing regimens.
- Formulation Development: The compound is involved in the development of various dosage forms, including tablets and orally disintegrating formulations, enhancing patient compliance and convenience.
- Comparative Effectiveness Research: It is used in studies comparing the effectiveness of different migraine treatments, helping healthcare providers make informed decisions about patient care.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.