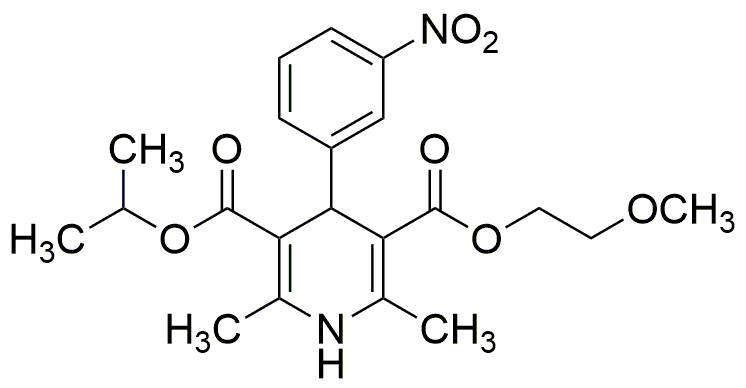
Nimodipine is widely utilized in research focused on:
- Treatment of Subarachnoid Hemorrhage: Nimodipine is primarily used in clinical settings to improve outcomes in patients who have suffered a subarachnoid hemorrhage, helping to prevent complications such as vasospasm.
- Neuroprotection: Researchers explore its neuroprotective properties, which may benefit conditions like stroke and traumatic brain injury by improving blood flow to the brain.
- Cardiovascular Research: The compound is studied for its effects on vascular smooth muscle, making it relevant in research on hypertension and other cardiovascular diseases.
- Pharmaceutical Development: Nimodipine serves as a model compound in drug formulation studies, particularly in the development of sustained-release formulations due to its unique pharmacokinetic profile.
- Investigating Calcium Channel Blockers: It is commonly used in studies examining the role of calcium channel blockers in various physiological and pathological processes, providing insights into their therapeutic potential.
General Information
Properties
Safety and Regulations
Applications
Nimodipine is widely utilized in research focused on:
- Treatment of Subarachnoid Hemorrhage: Nimodipine is primarily used in clinical settings to improve outcomes in patients who have suffered a subarachnoid hemorrhage, helping to prevent complications such as vasospasm.
- Neuroprotection: Researchers explore its neuroprotective properties, which may benefit conditions like stroke and traumatic brain injury by improving blood flow to the brain.
- Cardiovascular Research: The compound is studied for its effects on vascular smooth muscle, making it relevant in research on hypertension and other cardiovascular diseases.
- Pharmaceutical Development: Nimodipine serves as a model compound in drug formulation studies, particularly in the development of sustained-release formulations due to its unique pharmacokinetic profile.
- Investigating Calcium Channel Blockers: It is commonly used in studies examining the role of calcium channel blockers in various physiological and pathological processes, providing insights into their therapeutic potential.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.