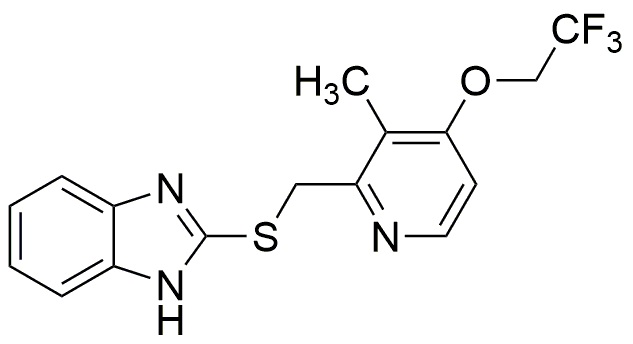
Lansoprazole sulfide is widely utilized in research focused on:
- Pharmaceutical Development: This compound is used in the formulation of medications aimed at treating gastrointestinal disorders, particularly those related to excess stomach acid. Its effectiveness in reducing acid secretion makes it a valuable component in proton pump inhibitors.
- Drug Metabolism Studies: Researchers leverage lansoprazole sulfide to study its metabolic pathways and interactions within the body. This helps in understanding how the drug is processed, which is crucial for optimizing dosing regimens.
- Formulation Chemistry: In the pharmaceutical industry, it serves as a model compound for developing new formulations that enhance drug stability and bioavailability, leading to more effective treatments.
- Comparative Efficacy Research: It is often used in studies comparing the efficacy of various acid-reducing agents, providing insights into how different compounds perform in clinical settings.
- Safety and Toxicology Assessments: The compound is important in evaluating the safety profiles of new drugs, helping to identify potential side effects and toxicological concerns early in the drug development process.
General Information
Properties
Safety and Regulations
Applications
Lansoprazole sulfide is widely utilized in research focused on:
- Pharmaceutical Development: This compound is used in the formulation of medications aimed at treating gastrointestinal disorders, particularly those related to excess stomach acid. Its effectiveness in reducing acid secretion makes it a valuable component in proton pump inhibitors.
- Drug Metabolism Studies: Researchers leverage lansoprazole sulfide to study its metabolic pathways and interactions within the body. This helps in understanding how the drug is processed, which is crucial for optimizing dosing regimens.
- Formulation Chemistry: In the pharmaceutical industry, it serves as a model compound for developing new formulations that enhance drug stability and bioavailability, leading to more effective treatments.
- Comparative Efficacy Research: It is often used in studies comparing the efficacy of various acid-reducing agents, providing insights into how different compounds perform in clinical settings.
- Safety and Toxicology Assessments: The compound is important in evaluating the safety profiles of new drugs, helping to identify potential side effects and toxicological concerns early in the drug development process.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.