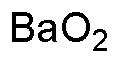Barium peroxide anhydrous is widely utilized in research focused on various applications:
- Oxidizing Agent: It serves as a powerful oxidizing agent in organic synthesis, facilitating reactions that require the introduction of oxygen into organic molecules.
- Bleaching Agent: In the textile and paper industries, it is employed as a bleaching agent, providing a more environmentally friendly alternative to chlorine-based bleaches.
- Water Treatment: Used in water treatment processes, it helps in the removal of impurities and enhances the quality of drinking water by oxidizing contaminants.
- Analytical Chemistry: Barium peroxide is utilized in analytical chemistry for the determination of various substances, including sulfides and other reducing agents, due to its high reactivity.
- Fireworks and Pyrotechnics: It is also a component in fireworks and pyrotechnic formulations, where it contributes to the production of bright white light and enhances the overall visual effects.
General Information
Properties
Safety and Regulations
Applications
Barium peroxide anhydrous is widely utilized in research focused on various applications:
- Oxidizing Agent: It serves as a powerful oxidizing agent in organic synthesis, facilitating reactions that require the introduction of oxygen into organic molecules.
- Bleaching Agent: In the textile and paper industries, it is employed as a bleaching agent, providing a more environmentally friendly alternative to chlorine-based bleaches.
- Water Treatment: Used in water treatment processes, it helps in the removal of impurities and enhances the quality of drinking water by oxidizing contaminants.
- Analytical Chemistry: Barium peroxide is utilized in analytical chemistry for the determination of various substances, including sulfides and other reducing agents, due to its high reactivity.
- Fireworks and Pyrotechnics: It is also a component in fireworks and pyrotechnic formulations, where it contributes to the production of bright white light and enhances the overall visual effects.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.