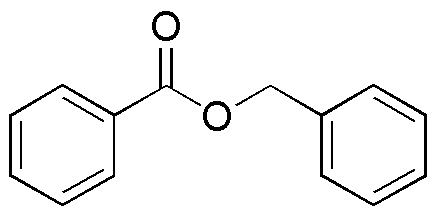
Benzyl benzoate is widely utilized in research focused on:
- Fragrance and Flavor Industry: This compound is commonly used as a fixative in perfumes and as a flavoring agent in food products, enhancing their aroma and taste.
- Pharmaceutical Applications: Benzyl benzoate serves as a solvent and carrier for various medicinal compounds, improving their efficacy and absorption in topical formulations.
- Pest Control: It is effective as an insect repellent and is often incorporated into formulations for controlling pests in agriculture, providing a safer alternative to harsher chemicals.
- Cosmetic Products: This chemical is used in lotions and creams for its moisturizing properties, making it a popular ingredient in skincare products.
- Laboratory Research: Benzyl benzoate is utilized as a reagent in organic synthesis and chemical research, facilitating various reactions due to its stability and compatibility with other compounds.
General Information
Properties
Safety and Regulations
Applications
Benzyl benzoate is widely utilized in research focused on:
- Fragrance and Flavor Industry: This compound is commonly used as a fixative in perfumes and as a flavoring agent in food products, enhancing their aroma and taste.
- Pharmaceutical Applications: Benzyl benzoate serves as a solvent and carrier for various medicinal compounds, improving their efficacy and absorption in topical formulations.
- Pest Control: It is effective as an insect repellent and is often incorporated into formulations for controlling pests in agriculture, providing a safer alternative to harsher chemicals.
- Cosmetic Products: This chemical is used in lotions and creams for its moisturizing properties, making it a popular ingredient in skincare products.
- Laboratory Research: Benzyl benzoate is utilized as a reagent in organic synthesis and chemical research, facilitating various reactions due to its stability and compatibility with other compounds.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.