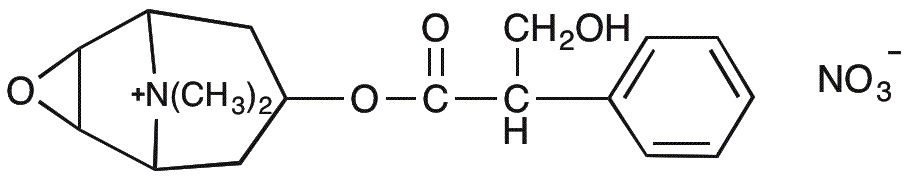
Scopolamine methyl nitrate is widely utilized in research focused on:
- Pharmaceutical Development: This compound is often used in the formulation of medications aimed at treating motion sickness and nausea, providing effective relief for patients in clinical settings.
- Neurological Studies: Researchers leverage its properties to study the effects of anticholinergic agents on the central nervous system, helping to advance understanding of various neurological disorders.
- Anesthesia Practices: In surgical environments, it is employed as an adjunct to anesthesia, enhancing patient comfort by reducing secretions and preventing nausea post-operation.
- Veterinary Medicine: Scopolamine methyl nitrate is also applied in veterinary practices to manage motion sickness in animals, ensuring their well-being during travel or procedures.
- Drug Interaction Research: Its unique chemical structure allows scientists to explore interactions with other medications, aiding in the development of safer and more effective therapeutic regimens.
General Information
Properties
Safety and Regulations
Applications
Scopolamine methyl nitrate is widely utilized in research focused on:
- Pharmaceutical Development: This compound is often used in the formulation of medications aimed at treating motion sickness and nausea, providing effective relief for patients in clinical settings.
- Neurological Studies: Researchers leverage its properties to study the effects of anticholinergic agents on the central nervous system, helping to advance understanding of various neurological disorders.
- Anesthesia Practices: In surgical environments, it is employed as an adjunct to anesthesia, enhancing patient comfort by reducing secretions and preventing nausea post-operation.
- Veterinary Medicine: Scopolamine methyl nitrate is also applied in veterinary practices to manage motion sickness in animals, ensuring their well-being during travel or procedures.
- Drug Interaction Research: Its unique chemical structure allows scientists to explore interactions with other medications, aiding in the development of safer and more effective therapeutic regimens.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.