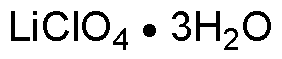Lithium perchlorate trihydrate is widely utilized in research focused on:
- Electrolytes in Batteries: This compound is commonly used as an electrolyte in lithium-ion batteries, enhancing their efficiency and stability. Its high ionic conductivity makes it ideal for improving battery performance in electric vehicles and portable electronics.
- Desiccants: Due to its hygroscopic nature, lithium perchlorate trihydrate is effective as a desiccant in various industrial applications, helping to control moisture levels in sensitive environments such as pharmaceuticals and electronics manufacturing.
- Analytical Chemistry: In laboratories, it serves as a reagent for the synthesis of other lithium compounds and is used in analytical methods to determine the presence of perchlorate ions in environmental samples.
- Solid-State Chemistry: Researchers utilize this compound in solid-state chemistry to study ionic conduction and develop new materials for advanced electronic devices, benefiting fields like nanotechnology and materials science.
- Hydration Studies: Its trihydrate form allows scientists to investigate hydration processes and thermodynamic properties, which are crucial for understanding various chemical reactions and processes in both academic and industrial settings.
General Information
Properties
Safety and Regulations
Applications
Lithium perchlorate trihydrate is widely utilized in research focused on:
- Electrolytes in Batteries: This compound is commonly used as an electrolyte in lithium-ion batteries, enhancing their efficiency and stability. Its high ionic conductivity makes it ideal for improving battery performance in electric vehicles and portable electronics.
- Desiccants: Due to its hygroscopic nature, lithium perchlorate trihydrate is effective as a desiccant in various industrial applications, helping to control moisture levels in sensitive environments such as pharmaceuticals and electronics manufacturing.
- Analytical Chemistry: In laboratories, it serves as a reagent for the synthesis of other lithium compounds and is used in analytical methods to determine the presence of perchlorate ions in environmental samples.
- Solid-State Chemistry: Researchers utilize this compound in solid-state chemistry to study ionic conduction and develop new materials for advanced electronic devices, benefiting fields like nanotechnology and materials science.
- Hydration Studies: Its trihydrate form allows scientists to investigate hydration processes and thermodynamic properties, which are crucial for understanding various chemical reactions and processes in both academic and industrial settings.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.