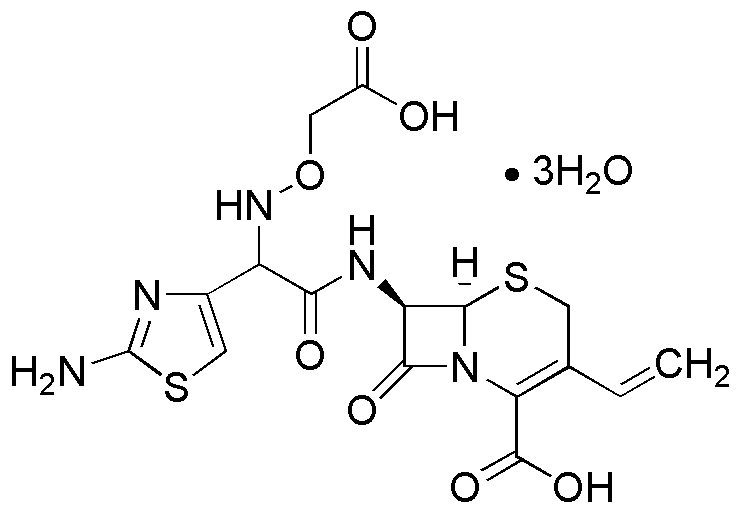
Cefixime trihydrate is widely utilized in research focused on:
- Antibiotic Development: This compound is a third-generation cephalosporin antibiotic, making it crucial in the development of new antibiotics to combat resistant bacterial strains.
- Clinical Applications: It is commonly prescribed for treating bacterial infections such as urinary tract infections and respiratory tract infections, providing effective treatment options for patients.
- Pharmaceutical Research: Researchers use cefixime trihydrate to study its pharmacokinetics and pharmacodynamics, helping to optimize dosing regimens and improve therapeutic outcomes.
- Formulation Studies: The compound is utilized in formulating oral medications, allowing for the development of stable and effective dosage forms for patient use.
- Microbial Resistance Studies: Cefixime trihydrate serves as a model compound in studies aimed at understanding microbial resistance mechanisms, aiding in the design of more effective antibiotics.
General Information
Properties
Safety and Regulations
Applications
Cefixime trihydrate is widely utilized in research focused on:
- Antibiotic Development: This compound is a third-generation cephalosporin antibiotic, making it crucial in the development of new antibiotics to combat resistant bacterial strains.
- Clinical Applications: It is commonly prescribed for treating bacterial infections such as urinary tract infections and respiratory tract infections, providing effective treatment options for patients.
- Pharmaceutical Research: Researchers use cefixime trihydrate to study its pharmacokinetics and pharmacodynamics, helping to optimize dosing regimens and improve therapeutic outcomes.
- Formulation Studies: The compound is utilized in formulating oral medications, allowing for the development of stable and effective dosage forms for patient use.
- Microbial Resistance Studies: Cefixime trihydrate serves as a model compound in studies aimed at understanding microbial resistance mechanisms, aiding in the design of more effective antibiotics.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.