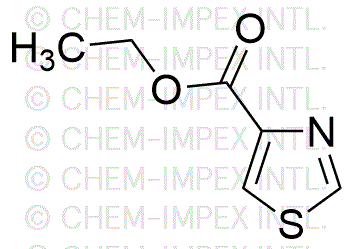
Ethyl 1,3-thiazole-4-carboxylate is widely utilized in research focused on:
- Pharmaceutical Development: This compound serves as a key intermediate in the synthesis of various pharmaceuticals, particularly those targeting bacterial infections. Its unique thiazole ring enhances biological activity compared to similar compounds.
- Agricultural Chemistry: It is used in the formulation of agrochemicals, including fungicides and herbicides, due to its effectiveness in controlling plant pathogens while being less toxic to beneficial organisms.
- Flavor and Fragrance Industry: Ethyl 1,3-thiazole-4-carboxylate is employed as a flavoring agent in food products, imparting a unique taste profile that enhances the sensory experience of consumers.
- Material Science: This compound is explored for its potential in creating novel materials, particularly in the development of polymers with enhanced properties, such as improved thermal stability and chemical resistance.
- Biochemical Research: It is utilized in various biochemical assays and studies, helping researchers understand metabolic pathways and enzyme activities, thus facilitating advancements in biochemistry and molecular biology.
General Information
Properties
Safety and Regulations
Applications
Ethyl 1,3-thiazole-4-carboxylate is widely utilized in research focused on:
- Pharmaceutical Development: This compound serves as a key intermediate in the synthesis of various pharmaceuticals, particularly those targeting bacterial infections. Its unique thiazole ring enhances biological activity compared to similar compounds.
- Agricultural Chemistry: It is used in the formulation of agrochemicals, including fungicides and herbicides, due to its effectiveness in controlling plant pathogens while being less toxic to beneficial organisms.
- Flavor and Fragrance Industry: Ethyl 1,3-thiazole-4-carboxylate is employed as a flavoring agent in food products, imparting a unique taste profile that enhances the sensory experience of consumers.
- Material Science: This compound is explored for its potential in creating novel materials, particularly in the development of polymers with enhanced properties, such as improved thermal stability and chemical resistance.
- Biochemical Research: It is utilized in various biochemical assays and studies, helping researchers understand metabolic pathways and enzyme activities, thus facilitating advancements in biochemistry and molecular biology.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.