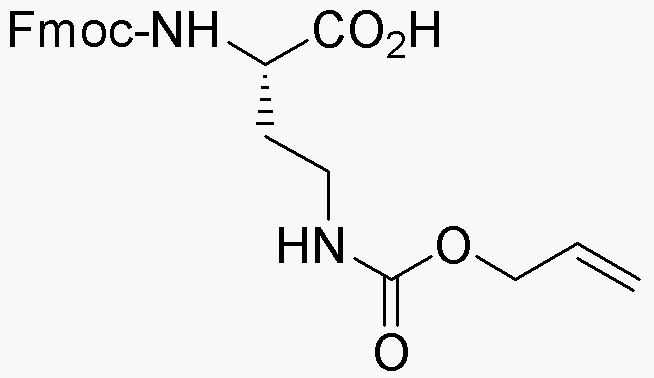
Na-Fmoc-Ng-allyloxycarbonyl-L-2,4-diaminobutyric acid is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protecting group in the synthesis of peptides, allowing for selective reactions without interfering with other functional groups. Its stability under various conditions makes it a preferred choice for chemists.
- Drug Development: In pharmaceutical research, it is used to design and modify bioactive compounds, enhancing their efficacy and selectivity, which is critical in developing new medications.
- Bioconjugation: The compound is effective in bioconjugation processes, where it helps attach biomolecules to surfaces or other molecules, facilitating the development of targeted drug delivery systems.
- Protein Engineering: Researchers employ this chemical in modifying proteins to study their functions and interactions, which is essential in fields such as biotechnology and molecular biology.
- Diagnostics: It plays a role in the development of diagnostic tools, where its ability to form stable conjugates aids in the detection of specific biomolecules, improving the accuracy of tests.
General Information
Properties
Safety and Regulations
Applications
Na-Fmoc-Ng-allyloxycarbonyl-L-2,4-diaminobutyric acid is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protecting group in the synthesis of peptides, allowing for selective reactions without interfering with other functional groups. Its stability under various conditions makes it a preferred choice for chemists.
- Drug Development: In pharmaceutical research, it is used to design and modify bioactive compounds, enhancing their efficacy and selectivity, which is critical in developing new medications.
- Bioconjugation: The compound is effective in bioconjugation processes, where it helps attach biomolecules to surfaces or other molecules, facilitating the development of targeted drug delivery systems.
- Protein Engineering: Researchers employ this chemical in modifying proteins to study their functions and interactions, which is essential in fields such as biotechnology and molecular biology.
- Diagnostics: It plays a role in the development of diagnostic tools, where its ability to form stable conjugates aids in the detection of specific biomolecules, improving the accuracy of tests.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.