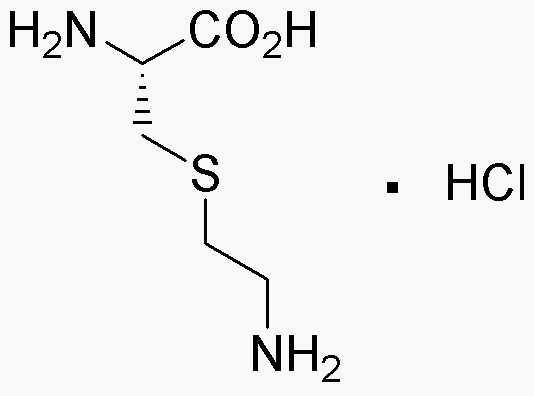S-(2-Aminoethyl-L-cysteine hydrochloride is widely utilized in research focused on:
- Biochemical Research: This compound serves as a valuable tool in studying protein interactions and enzyme activity due to its unique thiol group, which can form disulfide bonds, enhancing the understanding of protein folding and stability.
- Pharmaceutical Development: It is used in the synthesis of novel drugs, particularly those targeting neurological disorders, as it can act as a precursor for neurotransmitter synthesis, aiding in the development of therapeutic agents.
- Antioxidant Studies: Researchers leverage its antioxidant properties to investigate cellular protection mechanisms, providing insights into aging and disease processes, which can lead to the development of nutraceuticals.
- Cell Culture Applications: This compound is often incorporated into cell culture media to promote cell growth and viability, particularly in studies involving stem cells or primary cell lines, ensuring robust experimental results.
- Diagnostic Tools: It plays a role in the development of diagnostic assays, particularly in detecting specific biomarkers related to metabolic disorders, enhancing the accuracy of clinical diagnostics.
General Information
Properties
Safety and Regulations
Applications
S-(2-Aminoethyl-L-cysteine hydrochloride is widely utilized in research focused on:
- Biochemical Research: This compound serves as a valuable tool in studying protein interactions and enzyme activity due to its unique thiol group, which can form disulfide bonds, enhancing the understanding of protein folding and stability.
- Pharmaceutical Development: It is used in the synthesis of novel drugs, particularly those targeting neurological disorders, as it can act as a precursor for neurotransmitter synthesis, aiding in the development of therapeutic agents.
- Antioxidant Studies: Researchers leverage its antioxidant properties to investigate cellular protection mechanisms, providing insights into aging and disease processes, which can lead to the development of nutraceuticals.
- Cell Culture Applications: This compound is often incorporated into cell culture media to promote cell growth and viability, particularly in studies involving stem cells or primary cell lines, ensuring robust experimental results.
- Diagnostic Tools: It plays a role in the development of diagnostic assays, particularly in detecting specific biomarkers related to metabolic disorders, enhancing the accuracy of clinical diagnostics.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.