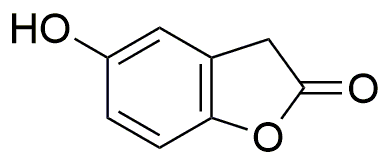
Homogentisic acid g-lactone is widely utilized in research focused on
- Biochemical Research: It plays a crucial role in studying metabolic pathways, particularly in the context of phenylalanine metabolism and its implications in disorders like alkaptonuria.
- Pharmaceutical Development: This compound is investigated for its potential therapeutic effects, especially in developing treatments for oxidative stress-related diseases.
- Food Industry: It is used as a natural antioxidant in food preservation, helping to enhance shelf life and maintain quality by preventing oxidative degradation.
- Cosmetic Formulations: The compound is explored for its skin-protective properties, making it a valuable ingredient in anti-aging and skin care products.
- Analytical Chemistry: It serves as a standard in various analytical methods, aiding in the quantification of related compounds in biological samples.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Homogentisic acid g-lactone is widely utilized in research focused on
- Biochemical Research: It plays a crucial role in studying metabolic pathways, particularly in the context of phenylalanine metabolism and its implications in disorders like alkaptonuria.
- Pharmaceutical Development: This compound is investigated for its potential therapeutic effects, especially in developing treatments for oxidative stress-related diseases.
- Food Industry: It is used as a natural antioxidant in food preservation, helping to enhance shelf life and maintain quality by preventing oxidative degradation.
- Cosmetic Formulations: The compound is explored for its skin-protective properties, making it a valuable ingredient in anti-aging and skin care products.
- Analytical Chemistry: It serves as a standard in various analytical methods, aiding in the quantification of related compounds in biological samples.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.