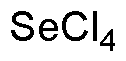Selenium (IV) chloride is widely utilized in research focused on:
- Synthesis of Organoselenium Compounds: This chemical serves as a key reagent in the production of various organoselenium compounds, which are valuable in pharmaceuticals and agrochemicals due to their unique biological activities.
- Analytical Chemistry: It is used in analytical methods for the detection and quantification of selenium in environmental samples, helping industries comply with safety regulations and environmental standards.
- Electronics Industry: Selenium (IV) chloride plays a role in the production of photoconductive materials, which are essential for manufacturing photocopiers and laser printers, enhancing their efficiency and performance.
- Research in Catalysis: This compound is explored as a catalyst in various chemical reactions, offering advantages such as increased reaction rates and selectivity, which can lead to more efficient industrial processes.
- Production of Selenium-Based Glass: It is utilized in the glass industry to produce selenium-containing glass, which has applications in optics and electronics, providing unique properties like improved light absorption.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Selenium (IV) chloride is widely utilized in research focused on:
- Synthesis of Organoselenium Compounds: This chemical serves as a key reagent in the production of various organoselenium compounds, which are valuable in pharmaceuticals and agrochemicals due to their unique biological activities.
- Analytical Chemistry: It is used in analytical methods for the detection and quantification of selenium in environmental samples, helping industries comply with safety regulations and environmental standards.
- Electronics Industry: Selenium (IV) chloride plays a role in the production of photoconductive materials, which are essential for manufacturing photocopiers and laser printers, enhancing their efficiency and performance.
- Research in Catalysis: This compound is explored as a catalyst in various chemical reactions, offering advantages such as increased reaction rates and selectivity, which can lead to more efficient industrial processes.
- Production of Selenium-Based Glass: It is utilized in the glass industry to produce selenium-containing glass, which has applications in optics and electronics, providing unique properties like improved light absorption.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.