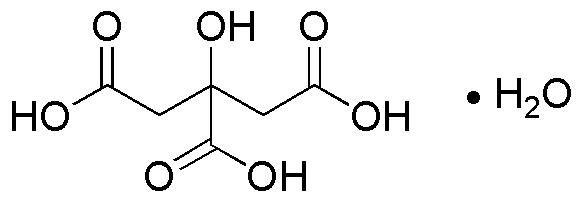
Citric acid monohydrate is widely utilized in research focused on:
- Food and Beverage Industry: It serves as a natural preservative and flavor enhancer, improving the taste and shelf life of products like soft drinks and canned goods.
- Pharmaceuticals: This compound is used in the formulation of medications, acting as a stabilizing agent and pH adjuster, which enhances the efficacy and safety of various drugs.
- Cosmetics and Personal Care: Citric acid monohydrate is incorporated in skincare products for its exfoliating properties, helping to improve skin texture and promote a youthful appearance.
- Cleaning Products: It is an effective chelating agent in household cleaners, helping to remove hard water stains and improve the effectiveness of detergents.
- Biotechnology: In laboratory settings, it is used as a buffer in biochemical assays, providing a stable pH environment that is crucial for accurate experimental results.
General Information
Properties
Safety and Regulations
Applications
Citric acid monohydrate is widely utilized in research focused on:
- Food and Beverage Industry: It serves as a natural preservative and flavor enhancer, improving the taste and shelf life of products like soft drinks and canned goods.
- Pharmaceuticals: This compound is used in the formulation of medications, acting as a stabilizing agent and pH adjuster, which enhances the efficacy and safety of various drugs.
- Cosmetics and Personal Care: Citric acid monohydrate is incorporated in skincare products for its exfoliating properties, helping to improve skin texture and promote a youthful appearance.
- Cleaning Products: It is an effective chelating agent in household cleaners, helping to remove hard water stains and improve the effectiveness of detergents.
- Biotechnology: In laboratory settings, it is used as a buffer in biochemical assays, providing a stable pH environment that is crucial for accurate experimental results.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.