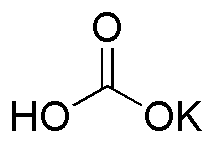
Potassium bicarbonate is widely utilized in research focused on:
- Food Industry: As a leavening agent, it helps baked goods rise by releasing carbon dioxide when heated, making it essential for producing light and fluffy textures in products like cakes and bread.
- Agriculture: Used as a mild fungicide, it effectively combats powdery mildew and other fungal diseases in crops, promoting healthier plants without the harsh chemicals found in many pesticides.
- Pharmaceuticals: It serves as an antacid to relieve heartburn and indigestion, providing a safe and effective way to neutralize stomach acid for patients.
- Environmental Applications: Employed in water treatment processes, it helps to neutralize acidic waters and improve pH levels, making it beneficial for maintaining aquatic ecosystems.
- Fire Extinguishers: As a key ingredient in some fire extinguishing agents, it effectively suppresses fires, particularly those involving flammable liquids, by releasing carbon dioxide when heated.
General Information
Properties
Safety and Regulations
Applications
Potassium bicarbonate is widely utilized in research focused on:
- Food Industry: As a leavening agent, it helps baked goods rise by releasing carbon dioxide when heated, making it essential for producing light and fluffy textures in products like cakes and bread.
- Agriculture: Used as a mild fungicide, it effectively combats powdery mildew and other fungal diseases in crops, promoting healthier plants without the harsh chemicals found in many pesticides.
- Pharmaceuticals: It serves as an antacid to relieve heartburn and indigestion, providing a safe and effective way to neutralize stomach acid for patients.
- Environmental Applications: Employed in water treatment processes, it helps to neutralize acidic waters and improve pH levels, making it beneficial for maintaining aquatic ecosystems.
- Fire Extinguishers: As a key ingredient in some fire extinguishing agents, it effectively suppresses fires, particularly those involving flammable liquids, by releasing carbon dioxide when heated.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.