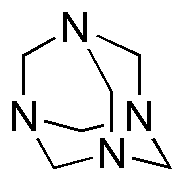
Hexamethylenetetramine is widely utilized in research focused on various practical applications:
- Pharmaceuticals: It serves as an important ingredient in the production of certain medications, particularly those used to treat urinary tract infections. Its ability to release formaldehyde in acidic environments aids in its antimicrobial properties.
- Industrial Adhesives: This compound is commonly used in the formulation of adhesives and sealants, providing strong bonding capabilities. Its thermal stability makes it suitable for high-temperature applications.
- Fuel Tablets: Hexamethylenetetramine is often found in solid fuel tablets for camping and military use. These tablets are lightweight, easy to store, and burn efficiently, making them ideal for outdoor cooking.
- Plastic and Resin Production: It acts as a curing agent in the production of certain plastics and resins, enhancing their durability and resistance to heat. This application is particularly valuable in the automotive and aerospace industries.
- Laboratory Reagent: In research settings, it is used as a reagent in various chemical syntheses and analyses, providing a reliable source of nitrogen and carbon in reactions.
General Information
Properties
Safety and Regulations
Applications
Hexamethylenetetramine is widely utilized in research focused on various practical applications:
- Pharmaceuticals: It serves as an important ingredient in the production of certain medications, particularly those used to treat urinary tract infections. Its ability to release formaldehyde in acidic environments aids in its antimicrobial properties.
- Industrial Adhesives: This compound is commonly used in the formulation of adhesives and sealants, providing strong bonding capabilities. Its thermal stability makes it suitable for high-temperature applications.
- Fuel Tablets: Hexamethylenetetramine is often found in solid fuel tablets for camping and military use. These tablets are lightweight, easy to store, and burn efficiently, making them ideal for outdoor cooking.
- Plastic and Resin Production: It acts as a curing agent in the production of certain plastics and resins, enhancing their durability and resistance to heat. This application is particularly valuable in the automotive and aerospace industries.
- Laboratory Reagent: In research settings, it is used as a reagent in various chemical syntheses and analyses, providing a reliable source of nitrogen and carbon in reactions.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.