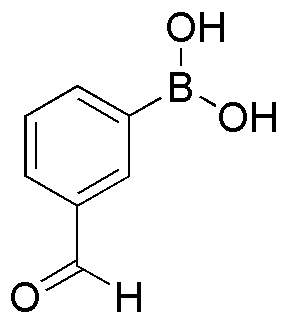
3-Boronobenzaldehyde is widely utilized in research focused on:
- Organic Synthesis: This compound serves as a key intermediate in the synthesis of various organic molecules, particularly in the development of pharmaceuticals and agrochemicals, allowing for the creation of complex structures efficiently.
- Cross-Coupling Reactions: It is commonly used in Suzuki-Miyaura coupling reactions, which are essential for forming carbon-carbon bonds. This application is crucial in the production of biaryl compounds, widely used in drug development.
- Fluorescent Probes: Researchers utilize 3-Boronobenzaldehyde in the design of fluorescent probes for biological imaging, enhancing the ability to visualize cellular processes in real-time.
- Material Science: This compound is applied in the development of advanced materials, such as polymers and nanomaterials, which have applications in electronics and coatings, providing improved performance characteristics.
- Chemical Sensors: It is also employed in the fabrication of chemical sensors, particularly for detecting specific analytes, which is vital in environmental monitoring and safety applications.
General Information
Properties
Safety and Regulations
Applications
3-Boronobenzaldehyde is widely utilized in research focused on:
- Organic Synthesis: This compound serves as a key intermediate in the synthesis of various organic molecules, particularly in the development of pharmaceuticals and agrochemicals, allowing for the creation of complex structures efficiently.
- Cross-Coupling Reactions: It is commonly used in Suzuki-Miyaura coupling reactions, which are essential for forming carbon-carbon bonds. This application is crucial in the production of biaryl compounds, widely used in drug development.
- Fluorescent Probes: Researchers utilize 3-Boronobenzaldehyde in the design of fluorescent probes for biological imaging, enhancing the ability to visualize cellular processes in real-time.
- Material Science: This compound is applied in the development of advanced materials, such as polymers and nanomaterials, which have applications in electronics and coatings, providing improved performance characteristics.
- Chemical Sensors: It is also employed in the fabrication of chemical sensors, particularly for detecting specific analytes, which is vital in environmental monitoring and safety applications.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.