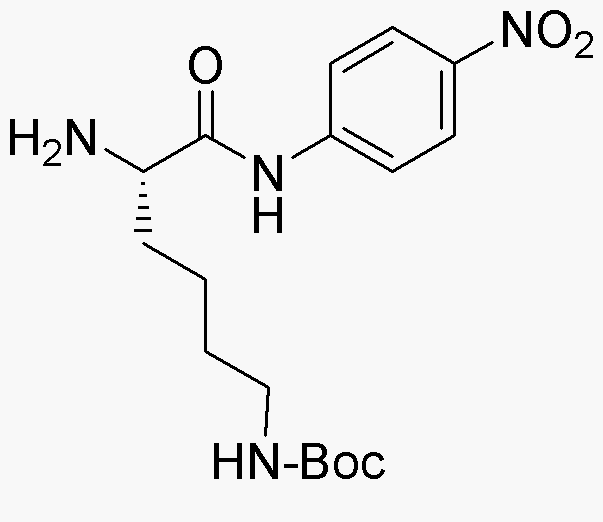
Ne-Boc-L-lysine 4-nitroanilide is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly for creating modified peptides with specific functional groups, enhancing the versatility of peptide-based drugs.
- Bioconjugation: It is employed in bioconjugation techniques, allowing researchers to attach biomolecules to surfaces or other molecules, which is crucial in developing targeted drug delivery systems.
- Fluorescent Probes: The compound can be used to create fluorescent probes for imaging applications, helping scientists visualize biological processes in real-time.
- Enzyme Inhibition Studies: It acts as a substrate or inhibitor in enzyme assays, aiding in the study of enzyme kinetics and the development of enzyme inhibitors for therapeutic purposes.
- Drug Development: Its unique structure allows for modifications that can lead to the development of new pharmaceuticals, particularly in the fields of oncology and infectious diseases.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Ne-Boc-L-lysine 4-nitroanilide is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly for creating modified peptides with specific functional groups, enhancing the versatility of peptide-based drugs.
- Bioconjugation: It is employed in bioconjugation techniques, allowing researchers to attach biomolecules to surfaces or other molecules, which is crucial in developing targeted drug delivery systems.
- Fluorescent Probes: The compound can be used to create fluorescent probes for imaging applications, helping scientists visualize biological processes in real-time.
- Enzyme Inhibition Studies: It acts as a substrate or inhibitor in enzyme assays, aiding in the study of enzyme kinetics and the development of enzyme inhibitors for therapeutic purposes.
- Drug Development: Its unique structure allows for modifications that can lead to the development of new pharmaceuticals, particularly in the fields of oncology and infectious diseases.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.