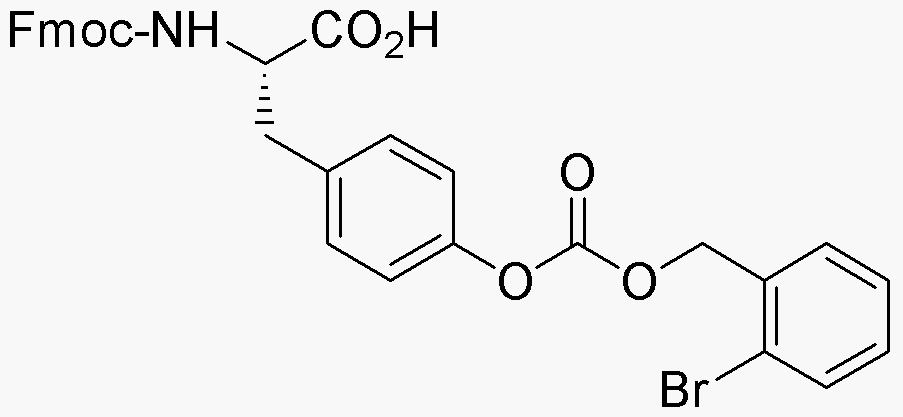
Fmoc-O-2-bromo-Z-L-tyrosine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly in solid-phase peptide synthesis (SPPS), allowing for the creation of complex peptide sequences.
- Drug Development: Its unique brominated structure can enhance the biological activity of peptide-based drugs, making it valuable in the pharmaceutical industry for developing new therapeutics.
- Bioconjugation: The compound can be used in bioconjugation processes to attach peptides to various biomolecules, facilitating the creation of targeted drug delivery systems.
- Research in Cancer Therapy: Due to its potential to modify peptide properties, it is being explored in cancer research for designing targeted therapies that can selectively attack cancer cells.
- Protein Engineering: The compound aids in the modification of proteins to study their structure and function, providing insights that can lead to advancements in biotechnology and molecular biology.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Fmoc-O-2-bromo-Z-L-tyrosine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly in solid-phase peptide synthesis (SPPS), allowing for the creation of complex peptide sequences.
- Drug Development: Its unique brominated structure can enhance the biological activity of peptide-based drugs, making it valuable in the pharmaceutical industry for developing new therapeutics.
- Bioconjugation: The compound can be used in bioconjugation processes to attach peptides to various biomolecules, facilitating the creation of targeted drug delivery systems.
- Research in Cancer Therapy: Due to its potential to modify peptide properties, it is being explored in cancer research for designing targeted therapies that can selectively attack cancer cells.
- Protein Engineering: The compound aids in the modification of proteins to study their structure and function, providing insights that can lead to advancements in biotechnology and molecular biology.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.