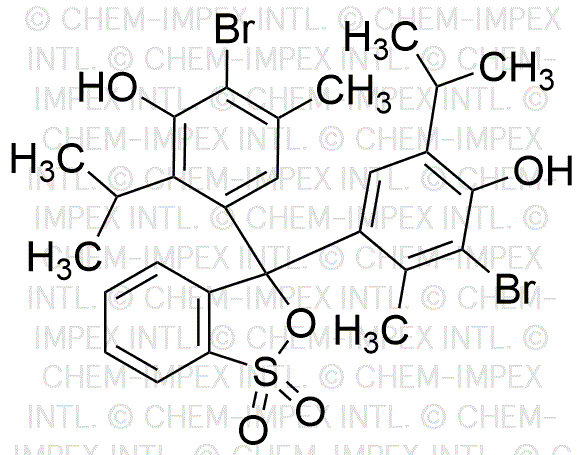
Bromothymol blue is widely utilized in research focused on:
- pH Indicator: Commonly used in laboratories to determine the acidity or alkalinity of solutions. It changes color from yellow in acidic conditions to blue in alkaline environments, making it a reliable choice for titrations and other chemical analyses.
- Biological Research: Employed in cell culture and microbiology to monitor pH changes in growth media, ensuring optimal conditions for cell viability and growth.
- Environmental Testing: Used in water quality testing to assess the pH of natural water bodies, helping researchers and environmentalists monitor ecosystem health.
- Education: A popular choice in educational settings for teaching students about acid-base chemistry through visual demonstrations, enhancing understanding of chemical concepts.
- Pharmaceuticals: Applied in drug formulation processes to ensure the stability and efficacy of medications by maintaining appropriate pH levels during production.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Bromothymol blue is widely utilized in research focused on:
- pH Indicator: Commonly used in laboratories to determine the acidity or alkalinity of solutions. It changes color from yellow in acidic conditions to blue in alkaline environments, making it a reliable choice for titrations and other chemical analyses.
- Biological Research: Employed in cell culture and microbiology to monitor pH changes in growth media, ensuring optimal conditions for cell viability and growth.
- Environmental Testing: Used in water quality testing to assess the pH of natural water bodies, helping researchers and environmentalists monitor ecosystem health.
- Education: A popular choice in educational settings for teaching students about acid-base chemistry through visual demonstrations, enhancing understanding of chemical concepts.
- Pharmaceuticals: Applied in drug formulation processes to ensure the stability and efficacy of medications by maintaining appropriate pH levels during production.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.