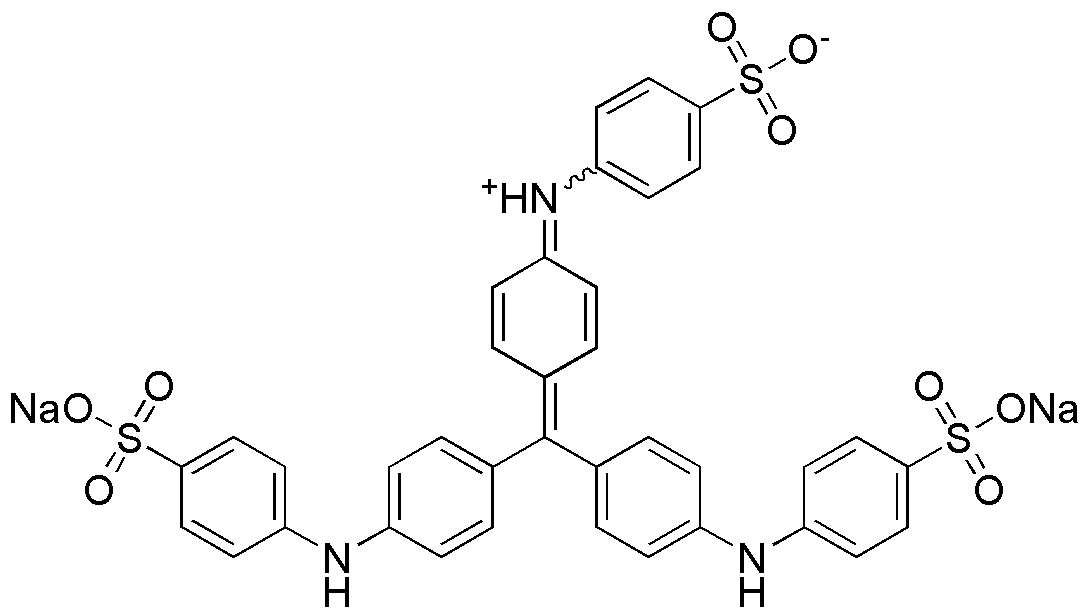
Methyl blue is widely utilized in research focused on:
- Biological Staining: Commonly used as a dye in microbiology and histology to stain cells and tissues, enhancing visibility under a microscope. This application is crucial for identifying cellular structures and diagnosing diseases.
- pH Indicator: Acts as a pH indicator in various chemical experiments, changing color to signify acidic or basic conditions. This property is particularly beneficial in educational settings and laboratory experiments where monitoring pH is essential.
- Redox Reactions: Employed in redox titrations due to its ability to undergo color changes based on oxidation states. This makes it a valuable tool for chemists in quantitative analysis and determining the concentration of substances.
- Textile Industry: Used as a dye in the textile industry to color fabrics, providing vibrant shades. Its stability and ease of application make it a preferred choice for manufacturers looking for reliable coloring agents.
- Environmental Monitoring: Applied in assessing water quality, as it can indicate the presence of certain pollutants. This application is vital for environmental scientists and regulatory agencies focused on maintaining safe water standards.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Methyl blue is widely utilized in research focused on:
- Biological Staining: Commonly used as a dye in microbiology and histology to stain cells and tissues, enhancing visibility under a microscope. This application is crucial for identifying cellular structures and diagnosing diseases.
- pH Indicator: Acts as a pH indicator in various chemical experiments, changing color to signify acidic or basic conditions. This property is particularly beneficial in educational settings and laboratory experiments where monitoring pH is essential.
- Redox Reactions: Employed in redox titrations due to its ability to undergo color changes based on oxidation states. This makes it a valuable tool for chemists in quantitative analysis and determining the concentration of substances.
- Textile Industry: Used as a dye in the textile industry to color fabrics, providing vibrant shades. Its stability and ease of application make it a preferred choice for manufacturers looking for reliable coloring agents.
- Environmental Monitoring: Applied in assessing water quality, as it can indicate the presence of certain pollutants. This application is vital for environmental scientists and regulatory agencies focused on maintaining safe water standards.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.