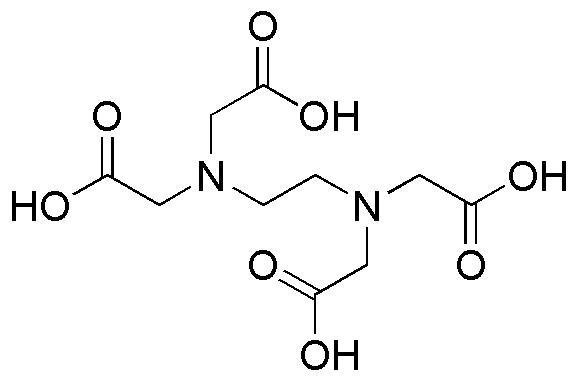
Ethylenediaminetetraacetic acid is widely utilized in research focused on:
- Metal Ion Chelation: This compound effectively binds to metal ions, making it invaluable in various industries such as water treatment, where it helps remove harmful heavy metals from water sources.
- Pharmaceutical Formulations: In the medical field, it is used to formulate drugs that require stabilization of metal ions, enhancing the efficacy and safety of medications.
- Biochemical Research: Researchers use it in laboratories to study enzyme activity and protein interactions, as it can help control metal ion concentrations in biological experiments.
- Food Preservation: It acts as a preservative in the food industry by preventing metal-catalyzed oxidation, which helps maintain the quality and shelf-life of food products.
- Cosmetic Products: Ethylenediaminetetraacetic acid is included in skincare formulations to improve product stability and enhance the effectiveness of active ingredients by chelating trace metals.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Ethylenediaminetetraacetic acid is widely utilized in research focused on:
- Metal Ion Chelation: This compound effectively binds to metal ions, making it invaluable in various industries such as water treatment, where it helps remove harmful heavy metals from water sources.
- Pharmaceutical Formulations: In the medical field, it is used to formulate drugs that require stabilization of metal ions, enhancing the efficacy and safety of medications.
- Biochemical Research: Researchers use it in laboratories to study enzyme activity and protein interactions, as it can help control metal ion concentrations in biological experiments.
- Food Preservation: It acts as a preservative in the food industry by preventing metal-catalyzed oxidation, which helps maintain the quality and shelf-life of food products.
- Cosmetic Products: Ethylenediaminetetraacetic acid is included in skincare formulations to improve product stability and enhance the effectiveness of active ingredients by chelating trace metals.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.