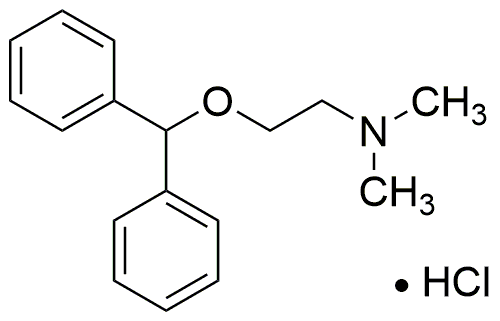
Diphenhydramine hydrochloride is widely utilized in research focused on:
- Allergy Relief: Commonly used in over-the-counter medications, it effectively alleviates symptoms of hay fever and other allergic reactions, making it a staple in the pharmaceutical industry.
- Sleep Aid: Due to its sedative properties, it is often included in sleep medications, providing a non-prescription option for individuals struggling with insomnia.
- Motion Sickness Prevention: Frequently used in travel medications, it helps prevent nausea and vomiting associated with motion sickness, benefiting travelers and those prone to discomfort.
- Cold and Flu Treatment: It is a common ingredient in combination cold medications, helping to relieve symptoms such as runny nose and sneezing, thus enhancing patient comfort during illness.
- Research Applications: In scientific studies, it serves as a model compound for investigating antihistaminic effects and understanding drug interactions, aiding researchers in developing new therapeutic agents.
General Information
Properties
Safety and Regulations
Applications
Diphenhydramine hydrochloride is widely utilized in research focused on:
- Allergy Relief: Commonly used in over-the-counter medications, it effectively alleviates symptoms of hay fever and other allergic reactions, making it a staple in the pharmaceutical industry.
- Sleep Aid: Due to its sedative properties, it is often included in sleep medications, providing a non-prescription option for individuals struggling with insomnia.
- Motion Sickness Prevention: Frequently used in travel medications, it helps prevent nausea and vomiting associated with motion sickness, benefiting travelers and those prone to discomfort.
- Cold and Flu Treatment: It is a common ingredient in combination cold medications, helping to relieve symptoms such as runny nose and sneezing, thus enhancing patient comfort during illness.
- Research Applications: In scientific studies, it serves as a model compound for investigating antihistaminic effects and understanding drug interactions, aiding researchers in developing new therapeutic agents.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.