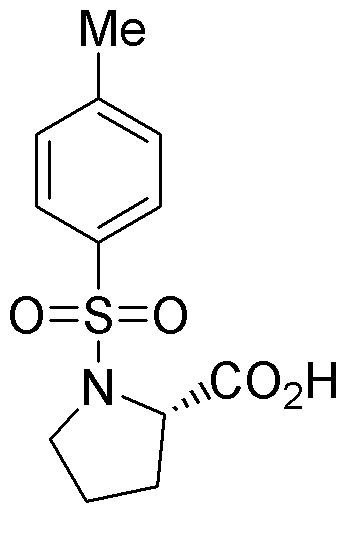
Tosyl-L-proline is widely utilized in research focused on:
- Peptide Synthesis: It serves as a valuable building block in the synthesis of peptides, enhancing the efficiency and selectivity of reactions.
- Drug Development: This compound is used in medicinal chemistry to develop new pharmaceuticals, particularly in creating compounds with improved bioactivity.
- Asymmetric Synthesis: Tosyl-L-proline is instrumental in asymmetric synthesis, allowing researchers to produce chiral molecules with high enantioselectivity.
- Catalysis: It acts as a catalyst in various organic reactions, providing advantages in reaction rates and yields compared to traditional catalysts.
- Biochemical Research: This compound is employed in biochemical studies to investigate enzyme mechanisms and protein interactions, offering insights into biological processes.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Tosyl-L-proline is widely utilized in research focused on:
- Peptide Synthesis: It serves as a valuable building block in the synthesis of peptides, enhancing the efficiency and selectivity of reactions.
- Drug Development: This compound is used in medicinal chemistry to develop new pharmaceuticals, particularly in creating compounds with improved bioactivity.
- Asymmetric Synthesis: Tosyl-L-proline is instrumental in asymmetric synthesis, allowing researchers to produce chiral molecules with high enantioselectivity.
- Catalysis: It acts as a catalyst in various organic reactions, providing advantages in reaction rates and yields compared to traditional catalysts.
- Biochemical Research: This compound is employed in biochemical studies to investigate enzyme mechanisms and protein interactions, offering insights into biological processes.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.