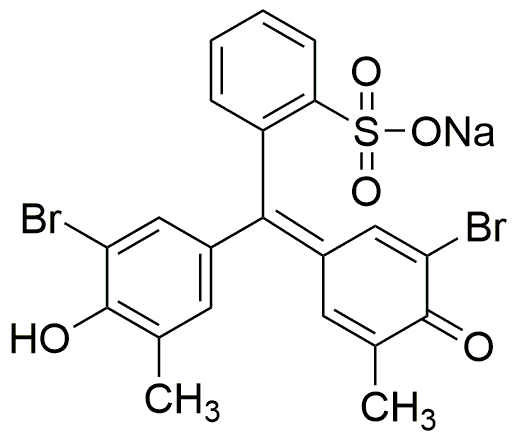
Bromocresol purple solution (0.04 wt. % in H2O) is widely utilized in research focused on:
- pH Indicator: This solution serves as a reliable pH indicator in various laboratory settings, providing clear color changes that help researchers determine the acidity or alkalinity of solutions quickly.
- Biochemical Assays: It is commonly used in biochemical assays to monitor enzymatic reactions, allowing scientists to track changes in pH that occur during metabolic processes.
- Microbiology: In microbiological studies, it assists in identifying bacterial growth conditions by indicating pH shifts in culture media, which can be crucial for optimizing growth parameters.
- Education: Educational institutions utilize this solution in chemistry and biology labs to teach students about acid-base reactions and the importance of pH in biological systems.
- Environmental Testing: It is applied in environmental science for testing water quality, helping to assess the health of aquatic ecosystems by monitoring pH levels.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Bromocresol purple solution (0.04 wt. % in H2O) is widely utilized in research focused on:
- pH Indicator: This solution serves as a reliable pH indicator in various laboratory settings, providing clear color changes that help researchers determine the acidity or alkalinity of solutions quickly.
- Biochemical Assays: It is commonly used in biochemical assays to monitor enzymatic reactions, allowing scientists to track changes in pH that occur during metabolic processes.
- Microbiology: In microbiological studies, it assists in identifying bacterial growth conditions by indicating pH shifts in culture media, which can be crucial for optimizing growth parameters.
- Education: Educational institutions utilize this solution in chemistry and biology labs to teach students about acid-base reactions and the importance of pH in biological systems.
- Environmental Testing: It is applied in environmental science for testing water quality, helping to assess the health of aquatic ecosystems by monitoring pH levels.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.