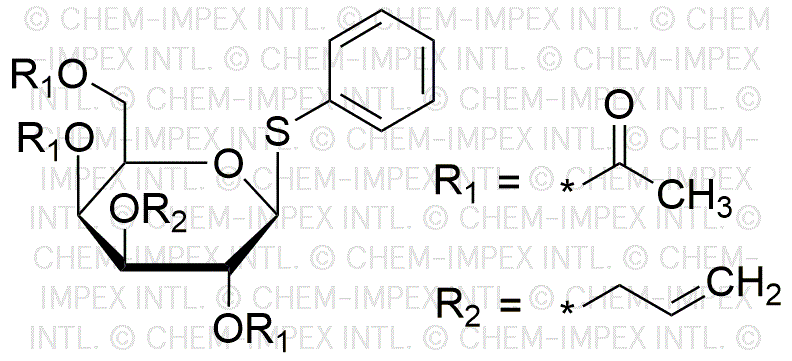Phenyl 2,4,6-tri-O-acetyl-3-O-allyl-1-thio-b-D-galactopyranoside is widely utilized in research focused on:
- Glycosylation Reactions: This compound serves as a glycosyl donor in organic synthesis, facilitating the formation of glycosidic bonds. This is particularly useful in the development of complex carbohydrates for pharmaceuticals.
- Drug Development: Its unique structure allows for modifications that can enhance the bioavailability and efficacy of drugs, making it valuable in the pharmaceutical industry for creating new therapeutic agents.
- Bioconjugation: The compound can be used in bioconjugation processes, linking biomolecules to drugs or imaging agents, which is essential in targeted therapy and diagnostic applications.
- Research in Carbohydrate Chemistry: It is a key reagent in the study of carbohydrate chemistry, aiding researchers in understanding glycan structures and their biological roles, which can lead to advancements in vaccine development.
- Food Industry Applications: The compound can be explored for use in food science, particularly in flavoring and preservation, leveraging its chemical properties to enhance food products.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Phenyl 2,4,6-tri-O-acetyl-3-O-allyl-1-thio-b-D-galactopyranoside is widely utilized in research focused on:
- Glycosylation Reactions: This compound serves as a glycosyl donor in organic synthesis, facilitating the formation of glycosidic bonds. This is particularly useful in the development of complex carbohydrates for pharmaceuticals.
- Drug Development: Its unique structure allows for modifications that can enhance the bioavailability and efficacy of drugs, making it valuable in the pharmaceutical industry for creating new therapeutic agents.
- Bioconjugation: The compound can be used in bioconjugation processes, linking biomolecules to drugs or imaging agents, which is essential in targeted therapy and diagnostic applications.
- Research in Carbohydrate Chemistry: It is a key reagent in the study of carbohydrate chemistry, aiding researchers in understanding glycan structures and their biological roles, which can lead to advancements in vaccine development.
- Food Industry Applications: The compound can be explored for use in food science, particularly in flavoring and preservation, leveraging its chemical properties to enhance food products.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.