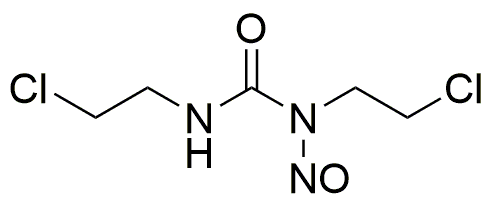
Carmustine is widely utilized in research focused on:
- Cancer Treatment: Primarily used as a chemotherapy agent for treating various types of cancers, including brain tumors and multiple myeloma. Its ability to cross the blood-brain barrier makes it particularly effective for central nervous system cancers.
- Veterinary Medicine: Employed in veterinary oncology for treating tumors in pets, providing an option for pet owners seeking advanced treatment for their animals.
- Research Applications: Utilized in laboratory settings to study cancer cell behavior and resistance mechanisms, helping researchers develop new therapeutic strategies.
- Drug Formulation: Incorporated in the development of combination therapies, enhancing the effectiveness of other anticancer drugs and reducing the likelihood of drug resistance.
- Pharmaceutical Development: Serves as a model compound in the synthesis of new nitrosourea derivatives, aiding in the discovery of novel anticancer agents with improved efficacy and safety profiles.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Carmustine is widely utilized in research focused on:
- Cancer Treatment: Primarily used as a chemotherapy agent for treating various types of cancers, including brain tumors and multiple myeloma. Its ability to cross the blood-brain barrier makes it particularly effective for central nervous system cancers.
- Veterinary Medicine: Employed in veterinary oncology for treating tumors in pets, providing an option for pet owners seeking advanced treatment for their animals.
- Research Applications: Utilized in laboratory settings to study cancer cell behavior and resistance mechanisms, helping researchers develop new therapeutic strategies.
- Drug Formulation: Incorporated in the development of combination therapies, enhancing the effectiveness of other anticancer drugs and reducing the likelihood of drug resistance.
- Pharmaceutical Development: Serves as a model compound in the synthesis of new nitrosourea derivatives, aiding in the discovery of novel anticancer agents with improved efficacy and safety profiles.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.