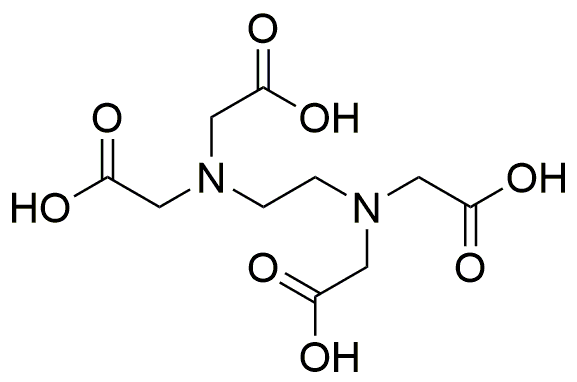
Ethylenediaminetetraacetic acid anhydrous, crystalline is widely utilized in research focused on:
- Metal Ion Chelation: This compound effectively binds to metal ions, making it invaluable in various industries such as water treatment, where it helps remove harmful heavy metals from wastewater.
- Pharmaceutical Formulations: In the pharmaceutical industry, it is used as a stabilizing agent in drug formulations, enhancing the solubility and bioavailability of certain medications.
- Laboratory Reagents: Commonly employed in biochemical assays, it serves as a reagent in laboratories for studying enzyme activity and metal ion interactions.
- Food Preservation: In the food industry, it acts as a preservative by chelating metal ions that can catalyze spoilage reactions, thus extending the shelf life of products.
- Cosmetic Applications: It is also found in cosmetic formulations, where it helps to stabilize products and improve their efficacy by preventing metal-induced degradation.
General Information
Properties
Safety and Regulations
Applications
Ethylenediaminetetraacetic acid anhydrous, crystalline is widely utilized in research focused on:
- Metal Ion Chelation: This compound effectively binds to metal ions, making it invaluable in various industries such as water treatment, where it helps remove harmful heavy metals from wastewater.
- Pharmaceutical Formulations: In the pharmaceutical industry, it is used as a stabilizing agent in drug formulations, enhancing the solubility and bioavailability of certain medications.
- Laboratory Reagents: Commonly employed in biochemical assays, it serves as a reagent in laboratories for studying enzyme activity and metal ion interactions.
- Food Preservation: In the food industry, it acts as a preservative by chelating metal ions that can catalyze spoilage reactions, thus extending the shelf life of products.
- Cosmetic Applications: It is also found in cosmetic formulations, where it helps to stabilize products and improve their efficacy by preventing metal-induced degradation.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.