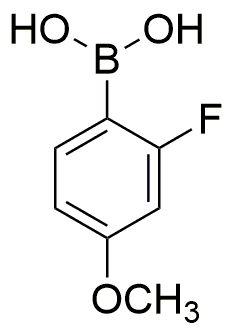
2-Fluoro-4-methoxyphenylboronic acid is widely utilized in research focused on:
- Pharmaceutical Development: This compound serves as a crucial building block in the synthesis of various pharmaceuticals, particularly in the development of drugs targeting cancer and other diseases. Its ability to form stable complexes with biomolecules enhances drug efficacy.
- Organic Synthesis: It is commonly used in Suzuki-Miyaura cross-coupling reactions, allowing chemists to create complex organic molecules. This application is vital in the production of agrochemicals and fine chemicals.
- Material Science: The compound is employed in the formulation of advanced materials, such as polymers and coatings, due to its unique properties that improve durability and performance in various applications.
- Bioconjugation: In biochemistry, it is utilized for labeling biomolecules, facilitating the study of protein interactions and cellular processes, which is essential for research in drug discovery and development.
- Analytical Chemistry: This boronic acid derivative is used in sensor technology for detecting sugars and other analytes, providing a sensitive and selective method for monitoring biological and environmental samples.
Informations générales
Propriétés
Sécurité et réglementation
Applications
2-Fluoro-4-methoxyphenylboronic acid is widely utilized in research focused on:
- Pharmaceutical Development: This compound serves as a crucial building block in the synthesis of various pharmaceuticals, particularly in the development of drugs targeting cancer and other diseases. Its ability to form stable complexes with biomolecules enhances drug efficacy.
- Organic Synthesis: It is commonly used in Suzuki-Miyaura cross-coupling reactions, allowing chemists to create complex organic molecules. This application is vital in the production of agrochemicals and fine chemicals.
- Material Science: The compound is employed in the formulation of advanced materials, such as polymers and coatings, due to its unique properties that improve durability and performance in various applications.
- Bioconjugation: In biochemistry, it is utilized for labeling biomolecules, facilitating the study of protein interactions and cellular processes, which is essential for research in drug discovery and development.
- Analytical Chemistry: This boronic acid derivative is used in sensor technology for detecting sugars and other analytes, providing a sensitive and selective method for monitoring biological and environmental samples.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.