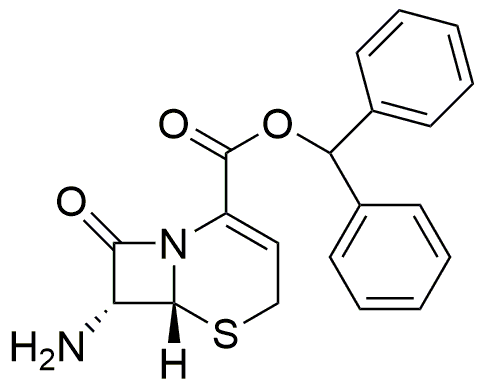
Diphenylmethyl 7b-amino-3-cephem-4-carboxylate is widely utilized in research focused on:
- Antibiotic Development: This compound serves as a precursor in the synthesis of novel cephalosporin antibiotics, which are crucial for treating bacterial infections. Its structure allows for modifications that enhance antibacterial activity.
- Pharmaceutical Formulations: It is used in the formulation of various pharmaceutical products, providing stability and improved bioavailability compared to other compounds in the same class.
- Research in Medicinal Chemistry: Researchers leverage its unique chemical properties to explore new therapeutic agents, particularly in combating antibiotic resistance, a growing concern in healthcare.
- Biochemical Studies: The compound is valuable in biochemical research for studying enzyme interactions and mechanisms, aiding in the understanding of drug action at the molecular level.
- Industrial Applications: Beyond pharmaceuticals, it finds applications in the development of agrochemicals, where its properties can be tailored for pest control solutions, offering an environmentally friendly alternative.
Informations générales
Propriétés
Sécurité et réglementation
Applications
Diphenylmethyl 7b-amino-3-cephem-4-carboxylate is widely utilized in research focused on:
- Antibiotic Development: This compound serves as a precursor in the synthesis of novel cephalosporin antibiotics, which are crucial for treating bacterial infections. Its structure allows for modifications that enhance antibacterial activity.
- Pharmaceutical Formulations: It is used in the formulation of various pharmaceutical products, providing stability and improved bioavailability compared to other compounds in the same class.
- Research in Medicinal Chemistry: Researchers leverage its unique chemical properties to explore new therapeutic agents, particularly in combating antibiotic resistance, a growing concern in healthcare.
- Biochemical Studies: The compound is valuable in biochemical research for studying enzyme interactions and mechanisms, aiding in the understanding of drug action at the molecular level.
- Industrial Applications: Beyond pharmaceuticals, it finds applications in the development of agrochemicals, where its properties can be tailored for pest control solutions, offering an environmentally friendly alternative.
Documents
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
Spécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
Certificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.
Numéro de catalogue
Numéro de lot/série
Fiches de données de sécurité (FDS)
La FDS fournit des informations de sécurité complètes sur la manipulation, le stockage et l’élimination du produit.
DownloadSpécifications du produit (PS)
Le PS fournit une description complète des propriétés du produit, notamment sa composition chimique, son état physique, sa pureté et les exigences de stockage. Il détaille également les plages de qualité acceptables et les applications prévues du produit.
DownloadCertificats d'analyse (COA)
Recherchez des certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot et de lot se trouvent sur l'étiquette d'un produit, après les mots « Lot » ou « Lot de fabrication ».
Numéro de catalogue
Numéro de lot/série
Certificats d'origine (COO)
Ce certificat d'exploitation confirme le pays dans lequel le produit a été fabriqué, et détaille également les matériaux et composants utilisés et s'il est issu de sources naturelles, synthétiques ou autres sources spécifiques. Ce certificat peut être requis pour les douanes, le commerce et la conformité réglementaire.