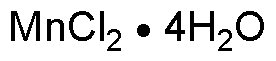Manganese(II) chloride tetrahydrate is widely utilized in research focused on various applications:
- Electrolyte in Batteries: This compound serves as an effective electrolyte in alkaline batteries, enhancing their performance and longevity. Its stable properties contribute to improved energy density.
- Animal Nutrition: It is commonly used as a dietary supplement in animal feed, providing essential manganese for growth and development, particularly in livestock and poultry.
- Catalyst in Chemical Reactions: Manganese(II) chloride tetrahydrate acts as a catalyst in several organic reactions, facilitating processes such as oxidation and polymerization, which are crucial in the chemical manufacturing industry.
- Water Treatment: This compound is applied in water treatment processes to remove impurities and control algae growth, making it valuable for maintaining water quality in industrial and municipal settings.
- Laboratory Reagent: It is frequently used in laboratories for various analytical procedures, including spectrophotometry and titrations, due to its reliable reactivity and availability.
General Information
Properties
Safety and Regulations
Applications
Manganese(II) chloride tetrahydrate is widely utilized in research focused on various applications:
- Electrolyte in Batteries: This compound serves as an effective electrolyte in alkaline batteries, enhancing their performance and longevity. Its stable properties contribute to improved energy density.
- Animal Nutrition: It is commonly used as a dietary supplement in animal feed, providing essential manganese for growth and development, particularly in livestock and poultry.
- Catalyst in Chemical Reactions: Manganese(II) chloride tetrahydrate acts as a catalyst in several organic reactions, facilitating processes such as oxidation and polymerization, which are crucial in the chemical manufacturing industry.
- Water Treatment: This compound is applied in water treatment processes to remove impurities and control algae growth, making it valuable for maintaining water quality in industrial and municipal settings.
- Laboratory Reagent: It is frequently used in laboratories for various analytical procedures, including spectrophotometry and titrations, due to its reliable reactivity and availability.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Numéro de catalogue
Numéro de lot/série
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Numéro de catalogue
Numéro de lot/série
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.