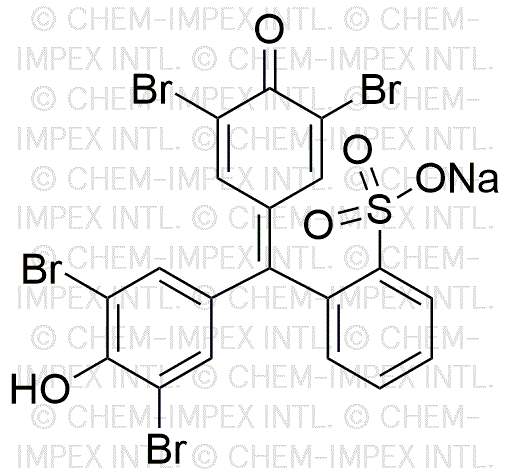
Bromophenol blue sodium salt is widely utilized in research focused on:
- pH Indicator: This compound is commonly used in laboratories as a pH indicator due to its clear color change from yellow to blue, making it ideal for visualizing pH levels in various solutions.
- Protein Electrophoresis: It serves as a tracking dye in gel electrophoresis, allowing researchers to monitor the progress of protein separation, which is crucial in biochemistry and molecular biology.
- Medical Diagnostics: In clinical laboratories, it is employed in assays to detect the presence of certain biomolecules, aiding in the diagnosis of various diseases.
- Environmental Testing: The compound can be used in environmental studies to assess the acidity of water samples, contributing to water quality monitoring and management.
- Educational Purposes: It is frequently used in educational settings for teaching purposes, helping students understand concepts related to pH and chemical reactions through hands-on experiments.
General Information
Properties
Safety and Regulations
Applications
Bromophenol blue sodium salt is widely utilized in research focused on:
- pH Indicator: This compound is commonly used in laboratories as a pH indicator due to its clear color change from yellow to blue, making it ideal for visualizing pH levels in various solutions.
- Protein Electrophoresis: It serves as a tracking dye in gel electrophoresis, allowing researchers to monitor the progress of protein separation, which is crucial in biochemistry and molecular biology.
- Medical Diagnostics: In clinical laboratories, it is employed in assays to detect the presence of certain biomolecules, aiding in the diagnosis of various diseases.
- Environmental Testing: The compound can be used in environmental studies to assess the acidity of water samples, contributing to water quality monitoring and management.
- Educational Purposes: It is frequently used in educational settings for teaching purposes, helping students understand concepts related to pH and chemical reactions through hands-on experiments.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Número de catálogo
Número de lote/lote
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.