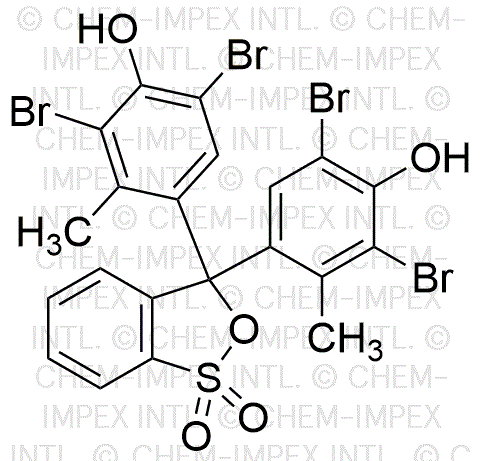
Bromocresol green sultone form is widely utilized in research focused on:
- pH Indicator in Laboratories: This compound serves as a reliable pH indicator in various laboratory settings, particularly for measuring pH levels in the range of 3.8 to 5.4. Its color change from yellow to blue provides a clear visual cue for researchers.
- Biochemical Assays: It is extensively used in biochemical assays to monitor pH changes during enzymatic reactions, helping scientists understand reaction kinetics and enzyme activity more effectively.
- Water Quality Testing: The compound is applied in environmental monitoring for assessing water quality. It helps determine the acidity of water samples, which is crucial for maintaining aquatic ecosystems and ensuring safe drinking water.
- Pharmaceutical Formulations: In the pharmaceutical industry, it is used as a pH indicator in formulations, ensuring that drugs maintain their efficacy and stability within the desired pH range.
- Educational Purposes: This chemical is commonly used in educational settings for teaching students about acid-base chemistry, providing a hands-on experience with pH indicators and their applications in real-world scenarios.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
Bromocresol green sultone form is widely utilized in research focused on:
- pH Indicator in Laboratories: This compound serves as a reliable pH indicator in various laboratory settings, particularly for measuring pH levels in the range of 3.8 to 5.4. Its color change from yellow to blue provides a clear visual cue for researchers.
- Biochemical Assays: It is extensively used in biochemical assays to monitor pH changes during enzymatic reactions, helping scientists understand reaction kinetics and enzyme activity more effectively.
- Water Quality Testing: The compound is applied in environmental monitoring for assessing water quality. It helps determine the acidity of water samples, which is crucial for maintaining aquatic ecosystems and ensuring safe drinking water.
- Pharmaceutical Formulations: In the pharmaceutical industry, it is used as a pH indicator in formulations, ensuring that drugs maintain their efficacy and stability within the desired pH range.
- Educational Purposes: This chemical is commonly used in educational settings for teaching students about acid-base chemistry, providing a hands-on experience with pH indicators and their applications in real-world scenarios.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.