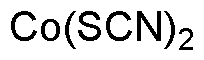Cobalt(II) thiocyanate is widely utilized in research focused on:
- Analytical Chemistry: Used as a reagent in various analytical techniques, it helps in the detection of certain metal ions, providing a reliable method for quality control in laboratories.
- Electrochemistry: Serves as an important component in the development of electrochemical sensors, enhancing the sensitivity and selectivity of measurements in environmental monitoring.
- Material Science: Employed in the synthesis of cobalt-based materials, which are crucial for developing high-performance batteries and catalysts, thus improving energy storage solutions.
- Biochemistry: Acts as a model compound for studying metal ion interactions in biological systems, aiding researchers in understanding enzyme mechanisms and metal transport in cells.
- Textile Industry: Utilized in dyeing processes, it provides vibrant colors and improved fabric properties, making it a valuable asset for manufacturers aiming for high-quality textile production.
General Information
Properties
Safety and Regulations
Applications
Cobalt(II) thiocyanate is widely utilized in research focused on:
- Analytical Chemistry: Used as a reagent in various analytical techniques, it helps in the detection of certain metal ions, providing a reliable method for quality control in laboratories.
- Electrochemistry: Serves as an important component in the development of electrochemical sensors, enhancing the sensitivity and selectivity of measurements in environmental monitoring.
- Material Science: Employed in the synthesis of cobalt-based materials, which are crucial for developing high-performance batteries and catalysts, thus improving energy storage solutions.
- Biochemistry: Acts as a model compound for studying metal ion interactions in biological systems, aiding researchers in understanding enzyme mechanisms and metal transport in cells.
- Textile Industry: Utilized in dyeing processes, it provides vibrant colors and improved fabric properties, making it a valuable asset for manufacturers aiming for high-quality textile production.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
Número de catálogo
Número de lote/lote
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Número de catálogo
Número de lote/lote
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.