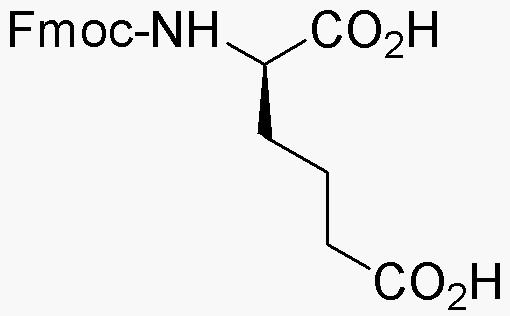
Fmoc-D-a-aminoadipic acid is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protective group in solid-phase peptide synthesis, allowing for the selective modification of amino acids without affecting the overall structure.
- Drug Development: It plays a crucial role in the design of peptide-based therapeutics, enhancing the stability and bioavailability of drug candidates.
- Biotechnology: Researchers use it in the development of novel biomaterials, leveraging its properties to create hydrogels and scaffolds for tissue engineering applications.
- Analytical Chemistry: The compound is utilized in various analytical techniques, including chromatography, to help separate and identify complex mixtures of peptides and proteins.
- Academic Research: It is frequently employed in laboratories for educational purposes, allowing students and researchers to explore peptide chemistry and its applications in a hands-on manner.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
Fmoc-D-a-aminoadipic acid is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a protective group in solid-phase peptide synthesis, allowing for the selective modification of amino acids without affecting the overall structure.
- Drug Development: It plays a crucial role in the design of peptide-based therapeutics, enhancing the stability and bioavailability of drug candidates.
- Biotechnology: Researchers use it in the development of novel biomaterials, leveraging its properties to create hydrogels and scaffolds for tissue engineering applications.
- Analytical Chemistry: The compound is utilized in various analytical techniques, including chromatography, to help separate and identify complex mixtures of peptides and proteins.
- Academic Research: It is frequently employed in laboratories for educational purposes, allowing students and researchers to explore peptide chemistry and its applications in a hands-on manner.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.