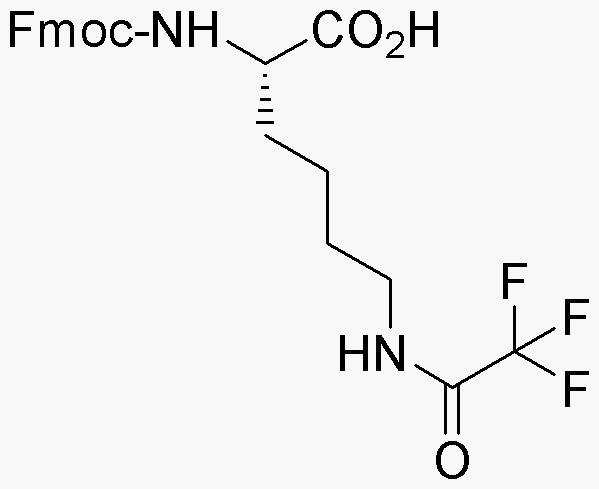
Na-Fmoc-Ne-trifluoroacetyl-L-lysine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly in solid-phase peptide synthesis, enabling researchers to create complex peptide sequences efficiently.
- Drug Development: Its unique protective groups allow for selective reactions, making it valuable in the pharmaceutical industry for developing new therapeutic peptides with enhanced stability and bioactivity.
- Bioconjugation: The trifluoroacetyl group can be used to facilitate bioconjugation processes, allowing for the attachment of various biomolecules, which is essential in creating targeted drug delivery systems.
- Protein Engineering: Researchers utilize this compound in the modification of lysine residues in proteins, which can help in studying protein interactions and functions, ultimately aiding in the design of novel proteins.
- Analytical Chemistry: It is also employed in analytical methods for characterizing peptides and proteins, providing insights into their structure and function, which is crucial for quality control in biopharmaceuticals.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
Na-Fmoc-Ne-trifluoroacetyl-L-lysine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly in solid-phase peptide synthesis, enabling researchers to create complex peptide sequences efficiently.
- Drug Development: Its unique protective groups allow for selective reactions, making it valuable in the pharmaceutical industry for developing new therapeutic peptides with enhanced stability and bioactivity.
- Bioconjugation: The trifluoroacetyl group can be used to facilitate bioconjugation processes, allowing for the attachment of various biomolecules, which is essential in creating targeted drug delivery systems.
- Protein Engineering: Researchers utilize this compound in the modification of lysine residues in proteins, which can help in studying protein interactions and functions, ultimately aiding in the design of novel proteins.
- Analytical Chemistry: It is also employed in analytical methods for characterizing peptides and proteins, providing insights into their structure and function, which is crucial for quality control in biopharmaceuticals.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.