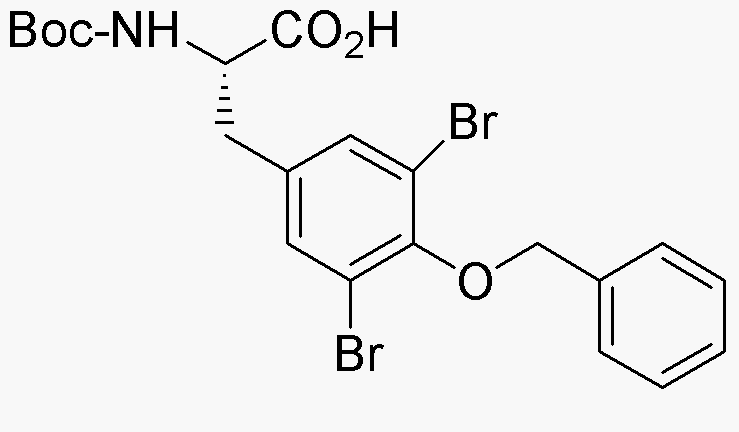
Boc-O-benzyl-3,5-dibromo-L-tyrosine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, allowing researchers to create complex molecules for drug development.
- Bioconjugation: It is employed in bioconjugation processes, where it helps attach biomolecules to surfaces or other molecules, enhancing the functionality of therapeutic agents.
- Drug Design: The unique bromine substitutions provide specific reactivity, making it advantageous in designing new pharmaceuticals with targeted properties.
- Research in Neuroscience: Its structural similarity to neurotransmitters allows for studies in neuropharmacology, aiding in the understanding of receptor interactions.
- Fluorescent Labeling: This compound can be used in fluorescent labeling techniques, helping visualize biological processes in real-time during experiments.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
Boc-O-benzyl-3,5-dibromo-L-tyrosine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, allowing researchers to create complex molecules for drug development.
- Bioconjugation: It is employed in bioconjugation processes, where it helps attach biomolecules to surfaces or other molecules, enhancing the functionality of therapeutic agents.
- Drug Design: The unique bromine substitutions provide specific reactivity, making it advantageous in designing new pharmaceuticals with targeted properties.
- Research in Neuroscience: Its structural similarity to neurotransmitters allows for studies in neuropharmacology, aiding in the understanding of receptor interactions.
- Fluorescent Labeling: This compound can be used in fluorescent labeling techniques, helping visualize biological processes in real-time during experiments.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.