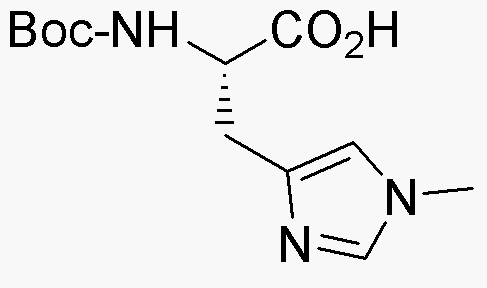
Boc-His(1-Me)-OH is widely utilized in research focused on
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly those containing histidine. Its protective Boc group allows for selective reactions, enhancing the efficiency of peptide assembly.
- Drug Development: In pharmaceutical research, it is used to create histidine-containing compounds that may have therapeutic effects, particularly in the development of drugs targeting metabolic disorders.
- Bioconjugation: The compound is employed in bioconjugation techniques, where it helps attach biomolecules to surfaces or other molecules, facilitating the development of targeted drug delivery systems.
- Research in Enzyme Inhibition: Boc-His(1-Me)-OH is useful in studying enzyme activity and inhibition, particularly in the context of histidine's role in enzyme catalysis, providing insights into biochemical pathways.
- Protein Engineering: It is applied in the field of protein engineering to modify histidine residues, allowing researchers to tailor protein properties for specific applications, such as improving stability or activity.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
Boc-His(1-Me)-OH is widely utilized in research focused on
- Peptide Synthesis: This compound serves as a key building block in the synthesis of peptides, particularly those containing histidine. Its protective Boc group allows for selective reactions, enhancing the efficiency of peptide assembly.
- Drug Development: In pharmaceutical research, it is used to create histidine-containing compounds that may have therapeutic effects, particularly in the development of drugs targeting metabolic disorders.
- Bioconjugation: The compound is employed in bioconjugation techniques, where it helps attach biomolecules to surfaces or other molecules, facilitating the development of targeted drug delivery systems.
- Research in Enzyme Inhibition: Boc-His(1-Me)-OH is useful in studying enzyme activity and inhibition, particularly in the context of histidine's role in enzyme catalysis, providing insights into biochemical pathways.
- Protein Engineering: It is applied in the field of protein engineering to modify histidine residues, allowing researchers to tailor protein properties for specific applications, such as improving stability or activity.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.