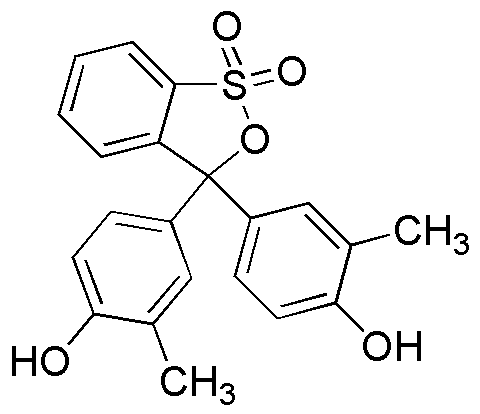
Cresol red is widely utilized in research focused on:
- pH Indicator: Commonly used in laboratories to measure pH levels in various solutions, providing a clear color change that helps researchers determine acidity or alkalinity.
- Biological Staining: Employed in histology and microbiology for staining tissues and cells, aiding in the visualization of structures under a microscope.
- Environmental Testing: Used in assessing water quality by indicating the pH of samples, which is crucial for monitoring aquatic ecosystems and ensuring safe drinking water.
- Pharmaceutical Development: Acts as a pH-sensitive dye in drug formulation studies, helping scientists understand how drugs behave in different pH environments, which is vital for effective delivery.
- Educational Purposes: Frequently included in chemistry education to demonstrate acid-base reactions and the concept of pH, making it a valuable tool for teaching students about chemical properties.
Información general
Propiedades
Seguridad y normativas
Aplicaciones
Cresol red is widely utilized in research focused on:
- pH Indicator: Commonly used in laboratories to measure pH levels in various solutions, providing a clear color change that helps researchers determine acidity or alkalinity.
- Biological Staining: Employed in histology and microbiology for staining tissues and cells, aiding in the visualization of structures under a microscope.
- Environmental Testing: Used in assessing water quality by indicating the pH of samples, which is crucial for monitoring aquatic ecosystems and ensuring safe drinking water.
- Pharmaceutical Development: Acts as a pH-sensitive dye in drug formulation studies, helping scientists understand how drugs behave in different pH environments, which is vital for effective delivery.
- Educational Purposes: Frequently included in chemistry education to demonstrate acid-base reactions and the concept of pH, making it a valuable tool for teaching students about chemical properties.
Documentos
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
Especificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
Certificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.
Número de catálogo
Número de lote/lote
Hojas de datos de seguridad (HDS)
La SDS proporciona información de seguridad completa sobre la manipulación, el almacenamiento y la eliminación del producto.
DownloadEspecificación del producto (PS)
La PS proporciona un desglose completo de las propiedades del producto, incluida la composición química, el estado físico, la pureza y los requisitos de almacenamiento. También detalla los rangos de calidad aceptables y las aplicaciones previstas del producto.
DownloadCertificados de análisis (COA)
Busque certificados de análisis (COA) ingresando el número de lote del producto. Los números de lote y de partida se pueden encontrar en la etiqueta de un producto después de las palabras "Lote" o "Lote".
Número de catálogo
Número de lote/lote
Certificados de origen (COO)
Este certificado de origen confirma el país en el que se fabricó el producto y también detalla los materiales y componentes utilizados en él y si se deriva de fuentes naturales, sintéticas u otras fuentes específicas. Este certificado puede ser necesario para cumplir con las normativas aduaneras, comerciales y regulatorias.