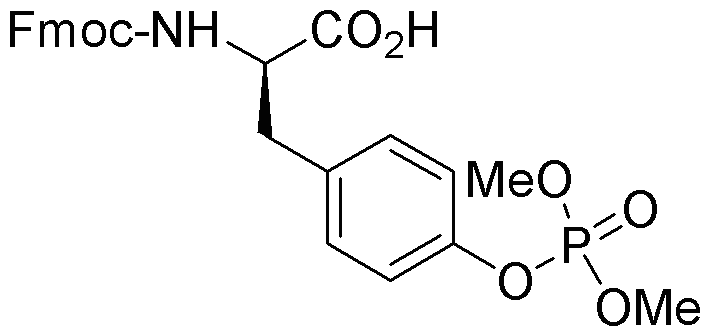
Fmoc-O-dimethylphospho-D-tyrosine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a crucial building block in the synthesis of phosphopeptides, which are important for studying protein interactions and signaling pathways.
- Drug Development: Its role in modifying amino acids allows researchers to create more effective therapeutic agents, particularly in targeting diseases related to cell signaling.
- Bioconjugation: The compound is used in the conjugation of biomolecules, enhancing the specificity and efficacy of drug delivery systems in cancer therapy.
- Analytical Chemistry: It aids in the development of analytical methods for detecting phosphorylated proteins, which are critical in understanding various biological processes.
- Biotechnology: The versatility of this compound supports advancements in biotechnology, particularly in the development of biosensors that detect specific biomolecules.
General Information
Properties
Safety and Regulations
Applications
Fmoc-O-dimethylphospho-D-tyrosine is widely utilized in research focused on:
- Peptide Synthesis: This compound serves as a crucial building block in the synthesis of phosphopeptides, which are important for studying protein interactions and signaling pathways.
- Drug Development: Its role in modifying amino acids allows researchers to create more effective therapeutic agents, particularly in targeting diseases related to cell signaling.
- Bioconjugation: The compound is used in the conjugation of biomolecules, enhancing the specificity and efficacy of drug delivery systems in cancer therapy.
- Analytical Chemistry: It aids in the development of analytical methods for detecting phosphorylated proteins, which are critical in understanding various biological processes.
- Biotechnology: The versatility of this compound supports advancements in biotechnology, particularly in the development of biosensors that detect specific biomolecules.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.