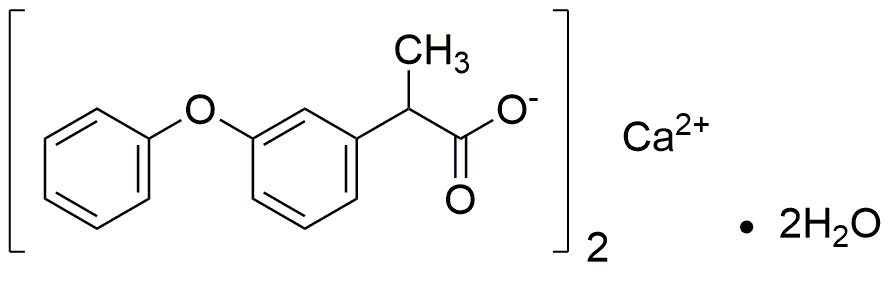
Fenoprofen calcium salt dihydrate is widely utilized in research focused on:
- Pain Management: This compound is commonly used in the pharmaceutical industry as a nonsteroidal anti-inflammatory drug (NSAID) to alleviate pain and inflammation associated with conditions like arthritis.
- Veterinary Medicine: It is applied in veterinary practices for treating pain and inflammation in animals, providing effective relief in various conditions.
- Drug Formulation: Researchers use this compound in the development of new drug formulations, leveraging its properties to enhance bioavailability and therapeutic effectiveness.
- Analytical Chemistry: Fenoprofen calcium salt dihydrate serves as a standard reference material in analytical laboratories, aiding in the calibration and validation of testing methods for drug analysis.
- Clinical Research: It is often included in clinical trials to evaluate its efficacy and safety, contributing valuable data for the advancement of pain management therapies.
General Information
Properties
Safety and Regulations
Applications
Fenoprofen calcium salt dihydrate is widely utilized in research focused on:
- Pain Management: This compound is commonly used in the pharmaceutical industry as a nonsteroidal anti-inflammatory drug (NSAID) to alleviate pain and inflammation associated with conditions like arthritis.
- Veterinary Medicine: It is applied in veterinary practices for treating pain and inflammation in animals, providing effective relief in various conditions.
- Drug Formulation: Researchers use this compound in the development of new drug formulations, leveraging its properties to enhance bioavailability and therapeutic effectiveness.
- Analytical Chemistry: Fenoprofen calcium salt dihydrate serves as a standard reference material in analytical laboratories, aiding in the calibration and validation of testing methods for drug analysis.
- Clinical Research: It is often included in clinical trials to evaluate its efficacy and safety, contributing valuable data for the advancement of pain management therapies.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.