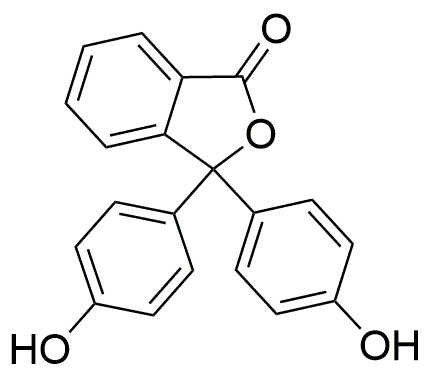
Phenolphthalein solution, 0.5 wt. % in ethanol: water (1:1) is widely utilized in research focused on:
- pH Indicator: Commonly used in titrations, it changes color from colorless to pink as the pH shifts from acidic to basic, making it an essential tool in chemistry labs.
- Pharmaceutical Applications: Employed in the formulation of certain medications, it helps in determining the pH of solutions, which is crucial for drug stability and efficacy.
- Educational Purposes: Frequently used in educational settings to demonstrate acid-base reactions, providing a visual representation that enhances learning for students in chemistry courses.
- Water Quality Testing: Utilized in environmental science to test the acidity or alkalinity of water samples, aiding in the monitoring of water quality for both industrial and recreational purposes.
- Cosmetic Formulations: In the cosmetic industry, it is used to ensure that products maintain a safe pH level, which is vital for skin compatibility and product performance.
General Information
Properties
Safety and Regulations
Applications
Phenolphthalein solution, 0.5 wt. % in ethanol: water (1:1) is widely utilized in research focused on:
- pH Indicator: Commonly used in titrations, it changes color from colorless to pink as the pH shifts from acidic to basic, making it an essential tool in chemistry labs.
- Pharmaceutical Applications: Employed in the formulation of certain medications, it helps in determining the pH of solutions, which is crucial for drug stability and efficacy.
- Educational Purposes: Frequently used in educational settings to demonstrate acid-base reactions, providing a visual representation that enhances learning for students in chemistry courses.
- Water Quality Testing: Utilized in environmental science to test the acidity or alkalinity of water samples, aiding in the monitoring of water quality for both industrial and recreational purposes.
- Cosmetic Formulations: In the cosmetic industry, it is used to ensure that products maintain a safe pH level, which is vital for skin compatibility and product performance.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.