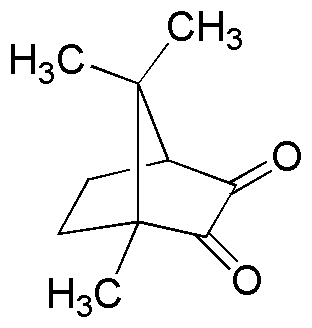
Camphorquinone is widely utilized in research focused on:
- Dental Applications: It serves as a photoinitiator in dental materials, particularly in light-cured resins. Its efficiency in initiating polymerization under light exposure enhances the durability and strength of dental restorations.
- Cosmetic Formulations: Commonly found in cosmetic products, it acts as a UV filter and stabilizer, helping to protect skin formulations from degradation due to light exposure, thus improving product longevity.
- Photopolymerization Processes: In the field of 3D printing and additive manufacturing, it is used to initiate polymerization in resin-based systems, allowing for precise control over the curing process and enhancing the quality of printed objects.
- Pharmaceutical Development: It plays a role in the synthesis of various pharmaceutical compounds, particularly in the development of drug delivery systems, where its photoinitiating properties can be leveraged to control the release of active ingredients.
- Research in Material Science: Utilized in the development of advanced materials, camphorquinone is involved in creating photo-responsive polymers, which can change properties when exposed to light, opening avenues for innovative applications in smart materials.
General Information
Properties
Safety and Regulations
Applications
Camphorquinone is widely utilized in research focused on:
- Dental Applications: It serves as a photoinitiator in dental materials, particularly in light-cured resins. Its efficiency in initiating polymerization under light exposure enhances the durability and strength of dental restorations.
- Cosmetic Formulations: Commonly found in cosmetic products, it acts as a UV filter and stabilizer, helping to protect skin formulations from degradation due to light exposure, thus improving product longevity.
- Photopolymerization Processes: In the field of 3D printing and additive manufacturing, it is used to initiate polymerization in resin-based systems, allowing for precise control over the curing process and enhancing the quality of printed objects.
- Pharmaceutical Development: It plays a role in the synthesis of various pharmaceutical compounds, particularly in the development of drug delivery systems, where its photoinitiating properties can be leveraged to control the release of active ingredients.
- Research in Material Science: Utilized in the development of advanced materials, camphorquinone is involved in creating photo-responsive polymers, which can change properties when exposed to light, opening avenues for innovative applications in smart materials.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.