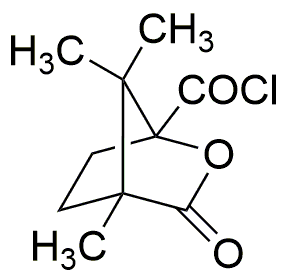
(1S)-(-)-Camphanic chloride is widely utilized in research focused on
- Synthesis of Pharmaceuticals: This compound serves as an important intermediate in the synthesis of various pharmaceutical agents, particularly in the development of analgesics and anti-inflammatory drugs.
- Flavor and Fragrance Industry: It is used to create specific flavor profiles and fragrances, providing a unique scent and taste that enhances food products and perfumes.
- Organic Synthesis: Researchers employ it in organic synthesis reactions, particularly in the formation of complex organic molecules, due to its reactive nature and ability to introduce functional groups.
- Chiral Auxiliary: As a chiral compound, it is utilized in asymmetric synthesis, helping chemists produce enantiomerically pure compounds, which is crucial in drug development.
- Research in Chemical Biology: It is used in studies involving enzyme catalysis and biomolecular interactions, aiding researchers in understanding biological processes at the molecular level.
General Information
Properties
Safety and Regulations
Applications
(1S)-(-)-Camphanic chloride is widely utilized in research focused on
- Synthesis of Pharmaceuticals: This compound serves as an important intermediate in the synthesis of various pharmaceutical agents, particularly in the development of analgesics and anti-inflammatory drugs.
- Flavor and Fragrance Industry: It is used to create specific flavor profiles and fragrances, providing a unique scent and taste that enhances food products and perfumes.
- Organic Synthesis: Researchers employ it in organic synthesis reactions, particularly in the formation of complex organic molecules, due to its reactive nature and ability to introduce functional groups.
- Chiral Auxiliary: As a chiral compound, it is utilized in asymmetric synthesis, helping chemists produce enantiomerically pure compounds, which is crucial in drug development.
- Research in Chemical Biology: It is used in studies involving enzyme catalysis and biomolecular interactions, aiding researchers in understanding biological processes at the molecular level.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.