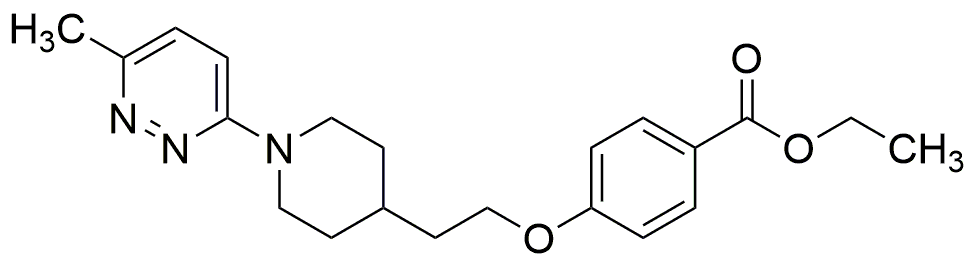
Pirodavir is widely utilized in research focused on:
- Antiviral Research: Pirodavir has shown effectiveness against various viral infections, making it a valuable compound in the development of antiviral therapies. Researchers are exploring its potential to combat respiratory viruses, which can lead to significant advancements in public health.
- Pharmaceutical Development: This compound is being investigated for its role in formulating new medications. Its unique structure allows for modifications that can enhance drug efficacy and reduce side effects, providing a competitive edge in drug design.
- In Vitro Studies: Pirodavir is frequently used in laboratory settings to study viral replication mechanisms. This application aids researchers in understanding how viruses operate, ultimately contributing to the development of targeted treatments.
- Combination Therapies: The compound is being evaluated for use in combination with other antiviral agents. This approach can enhance therapeutic outcomes by leveraging synergistic effects, which may lead to more effective treatment regimens.
- Clinical Trials: Pirodavir is currently involved in various clinical trials aimed at assessing its safety and efficacy in humans. This research is critical for bringing new antiviral solutions to market, addressing unmet medical needs.
General Information
Properties
Safety and Regulations
Applications
Pirodavir is widely utilized in research focused on:
- Antiviral Research: Pirodavir has shown effectiveness against various viral infections, making it a valuable compound in the development of antiviral therapies. Researchers are exploring its potential to combat respiratory viruses, which can lead to significant advancements in public health.
- Pharmaceutical Development: This compound is being investigated for its role in formulating new medications. Its unique structure allows for modifications that can enhance drug efficacy and reduce side effects, providing a competitive edge in drug design.
- In Vitro Studies: Pirodavir is frequently used in laboratory settings to study viral replication mechanisms. This application aids researchers in understanding how viruses operate, ultimately contributing to the development of targeted treatments.
- Combination Therapies: The compound is being evaluated for use in combination with other antiviral agents. This approach can enhance therapeutic outcomes by leveraging synergistic effects, which may lead to more effective treatment regimens.
- Clinical Trials: Pirodavir is currently involved in various clinical trials aimed at assessing its safety and efficacy in humans. This research is critical for bringing new antiviral solutions to market, addressing unmet medical needs.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.