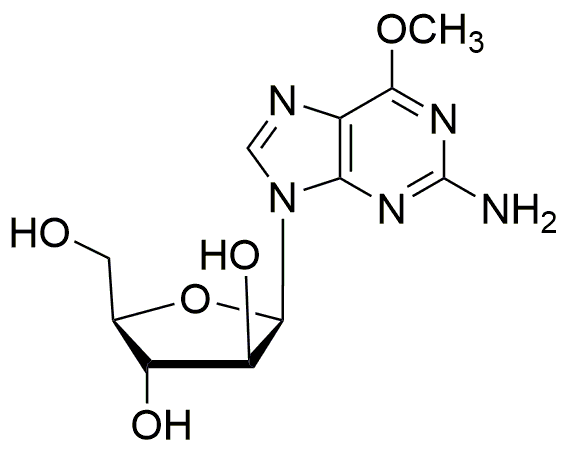
Nelarabine is widely utilized in research focused on:
- Oncology Treatments: This compound is primarily used in the treatment of certain types of leukemia, particularly T-cell acute lymphoblastic leukemia (T-ALL). Its effectiveness in targeting cancer cells makes it a vital option for patients who have not responded to other therapies.
- Combination Therapy: Nelarabine is often used in combination with other chemotherapeutic agents to enhance treatment efficacy. This synergistic approach can lead to improved outcomes for patients, especially in relapsed cases.
- Clinical Trials: Researchers are actively investigating nelarabine in clinical trials to explore its potential in treating other hematological malignancies. This ongoing research aims to expand its application and improve patient care.
- Pharmaceutical Development: The compound serves as a model for developing new purine analogs. Its unique structure provides insights into designing more effective drugs with fewer side effects.
- Patient Management: Nelarabine's specific action on T-cells allows healthcare providers to tailor treatment plans for patients with T-ALL, addressing individual needs and improving overall management of the disease.
General Information
Properties
Safety and Regulations
Applications
Nelarabine is widely utilized in research focused on:
- Oncology Treatments: This compound is primarily used in the treatment of certain types of leukemia, particularly T-cell acute lymphoblastic leukemia (T-ALL). Its effectiveness in targeting cancer cells makes it a vital option for patients who have not responded to other therapies.
- Combination Therapy: Nelarabine is often used in combination with other chemotherapeutic agents to enhance treatment efficacy. This synergistic approach can lead to improved outcomes for patients, especially in relapsed cases.
- Clinical Trials: Researchers are actively investigating nelarabine in clinical trials to explore its potential in treating other hematological malignancies. This ongoing research aims to expand its application and improve patient care.
- Pharmaceutical Development: The compound serves as a model for developing new purine analogs. Its unique structure provides insights into designing more effective drugs with fewer side effects.
- Patient Management: Nelarabine's specific action on T-cells allows healthcare providers to tailor treatment plans for patients with T-ALL, addressing individual needs and improving overall management of the disease.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.