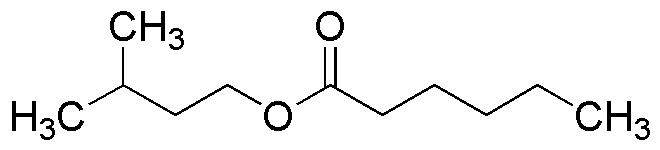
Isoamyl hexanoate is widely utilized in research focused on:
- Flavoring and Fragrance Industry: This compound is commonly used as a flavoring agent in food products and beverages, providing a pleasant fruity aroma reminiscent of bananas. Its natural appeal makes it a popular choice for manufacturers looking to enhance product appeal.
- Cosmetics and Personal Care: Due to its appealing scent, isoamyl hexanoate is incorporated into perfumes and personal care products. It helps create a desirable fragrance profile, making products more attractive to consumers.
- Aromatherapy: This chemical is used in essential oil blends for aromatherapy. Its fruity scent can promote relaxation and improve mood, making it a valuable ingredient in wellness products.
- Research Applications: In scientific studies, isoamyl hexanoate serves as a model compound for studying esterification reactions and the properties of esters, aiding researchers in understanding chemical behaviors and reactions.
- Food Preservation: Its antimicrobial properties can be beneficial in food preservation, helping to extend the shelf life of certain products while maintaining flavor integrity.
General Information
Properties
Safety and Regulations
Applications
Isoamyl hexanoate is widely utilized in research focused on:
- Flavoring and Fragrance Industry: This compound is commonly used as a flavoring agent in food products and beverages, providing a pleasant fruity aroma reminiscent of bananas. Its natural appeal makes it a popular choice for manufacturers looking to enhance product appeal.
- Cosmetics and Personal Care: Due to its appealing scent, isoamyl hexanoate is incorporated into perfumes and personal care products. It helps create a desirable fragrance profile, making products more attractive to consumers.
- Aromatherapy: This chemical is used in essential oil blends for aromatherapy. Its fruity scent can promote relaxation and improve mood, making it a valuable ingredient in wellness products.
- Research Applications: In scientific studies, isoamyl hexanoate serves as a model compound for studying esterification reactions and the properties of esters, aiding researchers in understanding chemical behaviors and reactions.
- Food Preservation: Its antimicrobial properties can be beneficial in food preservation, helping to extend the shelf life of certain products while maintaining flavor integrity.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.