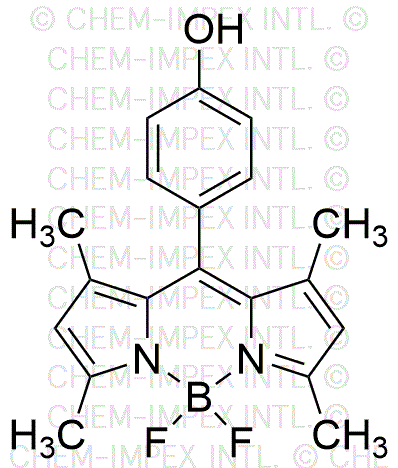
1,3,5,7-Tetramethyl-8-(4-hydroxyphenyl)BODIPY is widely utilized in research focused on:
- Fluorescent Imaging: This compound is an excellent fluorescent dye, making it ideal for biological imaging applications. Researchers use it to label cells and tissues, allowing for detailed visualization under a fluorescence microscope.
- Photodynamic Therapy: Its strong absorption properties make it suitable for photodynamic therapy in cancer treatment. By targeting cancer cells and activating the dye with light, it can help destroy malignant tissues while minimizing damage to surrounding healthy cells.
- Sensor Development: The compound is used in the development of sensors for detecting metal ions and other analytes. Its fluorescent properties change in response to specific environmental conditions, providing a reliable method for monitoring pollutants.
- Bioconjugation: Researchers leverage its reactive functional groups for bioconjugation, allowing for the attachment of biomolecules. This is particularly useful in creating targeted drug delivery systems and enhancing the efficacy of therapeutic agents.
- Solar Energy Applications: The compound's unique optical properties are being explored in the development of organic solar cells. Its ability to absorb light efficiently can improve the energy conversion efficiency of solar panels.
General Information
Properties
Safety and Regulations
Applications
1,3,5,7-Tetramethyl-8-(4-hydroxyphenyl)BODIPY is widely utilized in research focused on:
- Fluorescent Imaging: This compound is an excellent fluorescent dye, making it ideal for biological imaging applications. Researchers use it to label cells and tissues, allowing for detailed visualization under a fluorescence microscope.
- Photodynamic Therapy: Its strong absorption properties make it suitable for photodynamic therapy in cancer treatment. By targeting cancer cells and activating the dye with light, it can help destroy malignant tissues while minimizing damage to surrounding healthy cells.
- Sensor Development: The compound is used in the development of sensors for detecting metal ions and other analytes. Its fluorescent properties change in response to specific environmental conditions, providing a reliable method for monitoring pollutants.
- Bioconjugation: Researchers leverage its reactive functional groups for bioconjugation, allowing for the attachment of biomolecules. This is particularly useful in creating targeted drug delivery systems and enhancing the efficacy of therapeutic agents.
- Solar Energy Applications: The compound's unique optical properties are being explored in the development of organic solar cells. Its ability to absorb light efficiently can improve the energy conversion efficiency of solar panels.
Documents
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
Product Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.
*Catalog Number
*Lot Number
Safety Data Sheets (SDS)
The SDS provides comprehensive safety information on handling, storage, and disposal of the product.
DownloadProduct Specification (PS)
The PS provides a comprehensive breakdown of the product’s properties, including chemical composition, physical state, purity, and storage requirements. It also details acceptable quality ranges and the product's intended applications.
DownloadCertificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
*Catalog Number
*Lot Number
Certificates Of Origin (COO)
This COO confirms the country where the product was manufactured, and also details the materials and components used in it and whether it is derived from natural, synthetic, or other specific sources. This certificate may be required for customs, trade, and regulatory compliance.